INTRODUCTION
Fibrosis is a serious pathologic condition with excessive deposition of collagenous matrix that frequently results into a tissue stiffness and ultimately causes organ failure leading to death. As a result, it has been reported that about 45% of all deaths in developed countries are associated with some type of fibroproliferative diseases [1]. However, currently no effective therapies are available for the intervention of this devastating lung disease. In addition, the exact pathogenetic mechanism of tissue fibrosis is still largely elusive.
Idiopathic pulmonary fibrosis (IPF), a prototypic fibrotic disorder in the lung, is a progressive lung disease characterized by epithelial damage, fibroproliferative matrix deposition and parenchymal remodeling [2,3,4]. During the last decades, significant efforts have been directed to identify cells, mediators, and pathways responsible for pulmonary fibrosis. The myofibroblasts are known as the majors cells for increased accumulation of collagen and other extracelluar matrix in the lung, but the origin of these cells are not completely understood [5,6,7]. The role of transforming growth factor(TGF)-╬▓1 as a central mediator of tissue fibrosis was first recognized decades ago, numerous publications strongly still support its major role in a variety of fibrotic diseases [8,9,10,11]. Accordingly, TGF-╬▓1 and its signaling pathways have been regarded as attractive therapeutic targets to control fibroproliferative diseases including pulmonary fibrosis [12,13].
Recently, transgenic (Tg) mice overexpressing TGF-╬▓1 in the lung using lung-specific Clara cell 10 kDa (CC10) or surfactant protein C (SP-C) promoters were generated to define the direct in vivo effector function of TGF-╬▓1 [14,15]. In earlier studies, fetal-lethality was a serious problem to get live transgene expressing animals because TGF-╬▓1 plays a critical role in the development of airways [14]. Later, live lung-specific TGF-╬▓1 Tg mice were successfully generated using a tight regulated inducible system (CC10-tTS-rtTA-TGF-╬▓1), and these mice provided an exciting opportunity to look into the effector function of TGF-╬▓1 in the adult lung [15].
Intriguingly, the studies using CC10-tTS-rtTA-TGF-╬▓1 Tg mice first identified that TGF-╬▓1 induced epithelial cell death or injury response in the lung, followed by fibrotic tissue response [15]. These studies further demonstrated that apoptotic cellular response is a critical event for subsequent TGF-╬▓1-stimulated fibroproliferative repair response, suggesting controlling cellular apoptosis could be an effective therapeutic option for pulmonary fibrosis. The Tg approach was also successfully employed to identify a number of downstream mediators of TGF-╬▓1 in the lung, and some of these mediators, such as connective tissue growth factor and platelet-derived growth factor, were also shown to be effective therapeutic targets of pulmonary fibrosis [16].
Among downstream mediators of TGF-╬▓1 in the lung, it is interesting to note that amphiregulin (AR), an epidermal growth factor receptor (EGFR) ligand prominently induced by TGF-╬▓1, plays a critical role in pulmonary fibrosis [17]. Intervention of either AR expression or EGFR signaling significantly reduced TGF-╬▓1-induced pulmonary fibrosis, suggesting a critical role of EGFR signaling in this process [17]. The development and progression of pulmonary fibrosis are also significantly affected by other factors modulating the expression or activation of TGF-╬▓1 or its signaling pathways. Recent studies from our laboratory identified chitotriosidase (Chit1), a true chitinase (Cs) commonly detected in humans, was significantly associated with incidence of scleroderma-associated interstitial lung disease (SSc-ILD) and circulation levels of Chit1 were inversely correlated with lung function of SSc-ILD patients [18]. Interestingly, in vitro studies using a fibroblast cell line further identified that Chit1 sensitized TGF-╬▓1 signaling by enhancing TGF-╬▓1 receptor expression and activation of mitogen-activated protein kinase (MAPK)-Erk signaling.
These studies suggest that there are multiple factors (collectively designated as "modifiers" in this review) that significantly modulate the final outcome of TGF-╬▓1-induced tissue responses. These modifiers would be more effective and tolerable targets for the intervention of pulmonary fibrosis than simple TGF-╬▓1 blockers, because vital physiologic function of TGF-╬▓1 can be substantially preserved in this way. In this review, these exciting new approaches targeted to "modifiers" of TGF-╬▓1 for the intervention of pulmonary fibrosis are being highlighted with general introduction of the role and effector function of TGF-╬▓1 in the pathogenesis of pulmonary fibrosis.
TGF-╬▓1: CENTRAL MEDIATOR OF IPF
TGF-╬▓1 is believed to play an important role in the pathogenesis of IPF because it is expressed in an exaggerated fashion in IPF where, in contrast to controls, a sizable percentage is biologically active [19,20,21]. The important role that TGF-╬▓1 may play in this disorder can be seen in studies that demonstrate that TGF-╬▓1 is a critical mediator of pulmonary fibrosis after bleomycin injury [22,23] and that high dose adenoviral TGF-╬▓1 transfer causes progressive pulmonary fibrosis in vivo [24,25] and IPF-like fibroblastic foci in in vitro explants [21]. Interestingly, the apposition of apoptosis, fibrosis and exaggerated TGF-╬▓1 expression is well documented in IPF [26,27,28,29], and recent studies with TGF-╬▓1 Tg mice highlighted the importance of epithelial cell apoptosis in the pathogenesis of pulmonary fibrosis [15]. All these studies suggest that TGF-╬▓1 plays a critical role in pulmonary fibrosis through regulation of injury and repair responses. However, the factors that control these TGF-╬▓1 responses and the genetic and other processes that allow TGF-╬▓1 to contribute to the pathogenesis of fibrosis are still largely elusive and need to be further defined in future studies.
TGF-╬▓1 AND ITS EFFECTOR FUNCTION AND GENETIC MODIFIERS
TGF-╬▓1 family proteins are multifunctional cytokines that play pivotal roles in diverse biologic processes including cell growth and survival, cell and tissue differentiation, development, inflammation, immunity and tissue remodeling and repair.
On superficial analysis, TGF-╬▓1 can be accurately described as a healing molecule that manifests anti-inflammatory and fibrotic effects while inducing wound healing. On closer analysis, it is clear that this is only partially correct and that the effector profile of TGF-╬▓1 can appear confusing and even contradictory [30,31,32,33]. This can be seen in inflammation where TGF-╬▓1 has important anti-inflammatory effects in some settings [34,35] and proinflammatory effects in others [35,36]. This is also seen in oncogenesis where TGF-╬▓1 inhibits tumor cell growth while enhancing tumor migration and invasion [32]. TGF-╬▓1 is essential for wound healing, stimulates matrix molecule deposition and angiogenesis and is an essential mediator of the pathologic scaring in fibrotic disorders [22,23,30,37,38,39]. On the other hand, TGF-╬▓1 can also induce tissue injury [40], induce cellular apoptosis, decrease epithelialization and inhibit wound healing [31,33,41,42,43]. The complexity of TGF-╬▓1 effector functions can be attributed to a number of items. In particular, the effects of TGF-╬▓1 proteins vary with the state of activation and differentiation of the target cells and the presence of other stimuli in the local microenvironment. The diversity in TGF-╬▓1 receptor expression, depending on the type of cells, significantly impacts the cellular and tissue responses of TGF-╬▓1. However, the mechanism causing the diversity in cellular and tissue effects of TGF-╬▓1 is still largely elusive and needs to be addressed in future studies.
To define its effector functions in the lung, we developed Tg mice in which bioactive TGF-╬▓1 was inducibly overexpressed in the lung [15]. Studies using these mice demonstrated that Tg TGF-╬▓1 simultaneously induces tissue injury (apoptosis) and activates healing (fibrosis) and that the apoptosis is an obligatory prerequisite for fibrosis [15]. Recent studies demonstrate that the ultimate TGF-╬▓1-induced tissue response is dependent on murine genetic background and that these outcomes can be explained by the relative balance of injury, proteolysis, and fibrogenesis [44,45]. The strain dependency of TGF-╬▓1-stimulated pulmonary fibrosis strongly support the presence of genetically defined "modifiers" that significantly modulate the TGF-╬▓1-stimulated tissue response. In support of this observation, there is similar strain dependency in the radiation- or asbestos-induced pulmonary fibrosis [46,47]. Identification of these genetic modifiers will provide an exciting opportunity to understand the mechanism of TGF-╬▓-induced fibrosis, that will lead to the discovery of novel and effective therapeutic targets with less undesirable effects for the treatment of pulmonary fibrosis.
TGF-╬▓1 SIGNALING AND CROSSTALK WITH EGFR SIGNALING
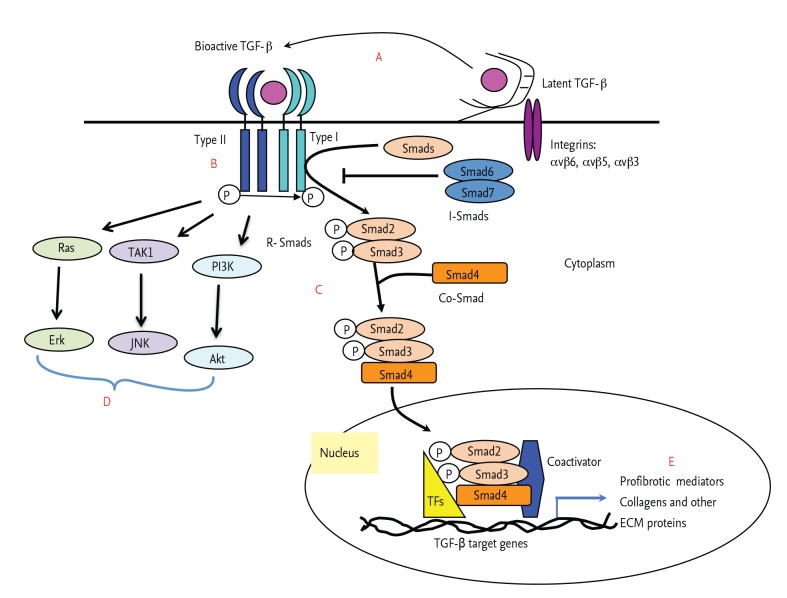
It has been well demonstrated that TGF-╬▓1 binds and signals primarily through heterodimers of TGF-╬▓1 receptor type I and type II complex that subsequently activates the cascade of Smads (receptor-regulated Smads [R-Smads; Smads1, 2, 3, 5] and common mediator Smads [Co-Smads; Smad4]). The activated Smads complexes are transported to the nucleus and plays an essential role in the expression of TGF-╬▓1 target genes together with other transcription factors such as CREB1-binding protein and p300. In addition, inhibitory Smads (such as inhibitory Smads6 and 7) and interacting molecules (such as SARA or Ski/SnoN) are associated with fine-tuning of this signaling pathway (Fig. 1) [48,49]. This is called canonical or Smad-dependent signaling pathways of TGF-╬▓1 and regarded as a major pathway of TGF-╬▓1 signaling [50]. However, a number of other signaling pathways are also implicated in the tissue responses stimulated by TGF-╬▓1. The MAPK/Erk, p38, c-Jun N-terminal kinase, nuclear factor-╬║╬▓, and phosphatidylinositol 3-kinase signaling pathways are differently activated by TGF-╬▓1 stimulation, depending on the type of cells and microenvironment. These noncanonical (Smad-independent) pathways of TGF-╬▓1 also significantly contribute to the diverse biological function of TGF-╬▓1 (Fig. 1) [50].
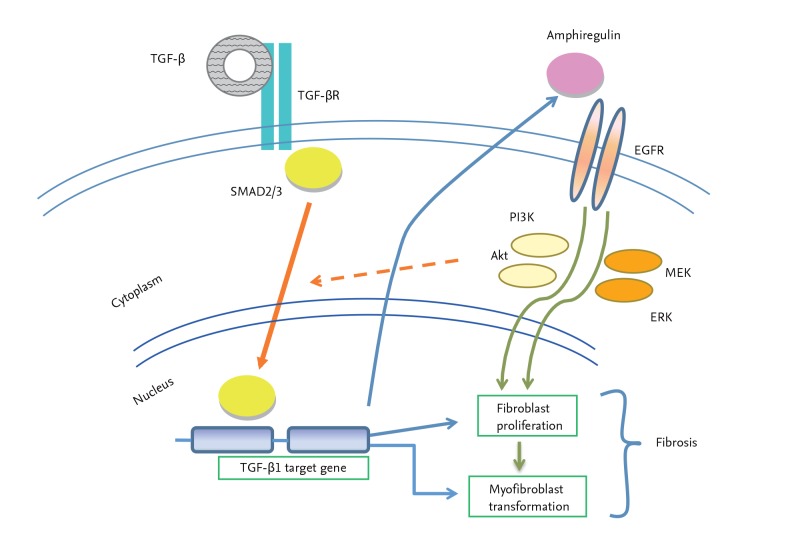
Interestingly, recent studies using TGF-╬▓1 Tg mice demonstrated that TGF-╬▓1 overexpression in the lung significantly induces AR, a EGFR ligand, and AR-stimulated EGFR signaling is also crucial for both canonical and noncanonical TGF-╬▓1 signaling (Fig. 2) [17]. In this study, siRNA silencing of AR or chemical ablation of EGFR signaling significantly decreased TGF-╬▓1-stimulated fibroblast proliferation and myofibroblast transformation, suggesting a crucial roles of AR and EGFR signaling in TGF-╬▓1-stimulated fibrotic tissue response in the lung.
These studies suggested that multiple signaling pathways are meticulously connected to each other, presumably for the fine-tuning of cellular responses stimulated by TGF-╬▓1. The profibrotic role of EGFR signaling was also elegantly demonstrated in a bleomycin-stimulated model of pulmonary fibrosis using gefitinib, a specific kinase inhibitor of EGFR signaling [51]. Since EGF is known to stimulate fibroblast proliferation [52], it is interesting to speculate a potential synergistic interaction between TGF-╬▓1 and EGFR signaling in fibroblast proliferation and myofibroblast transformation. Similar crosstalk among Smads2/3, EGFR and p53 pathways was reported in the expression of TGF-╬▓1-induced fibrotic target genes [53]. However, whether and how these two major cellular signaling pathways are being coordinated in the development and progression of pulmonary fibrosis remains to be determined in future studies. It will be an exciting field of future investigation to identify molecules linking different signaling pathways, such as AR, since these molecules could be effective and novel alternative therapeutic targets that regulate complex tissue phenotypes in which multiple signaling pathways are implicated.
CHITOTRIOSIDASE IN SCLERODERMA: A MODIFIER OF TGF-╬▓1 SIGNALING
The GH18 gene family contains Cs that bind and cleave chitin and chitinase-like proteins (CLP) that bind but do not cleave the chitin polysaccharide. These C/CLP are found across species from lower life forms (archea, prokaryotes, eukaryotes) to man. The nature of their contributions has been enigmatic because chitin is the only documented substrate of Cs, chitin and chitin synthase do not exist in mammals, and higher life forms do not use chitin as a nutrient [54,55]. Only acidic mammalian chitinase and chitotriosidase (chitinase 1; Chit1) are Cs. All of the rest are CLP (also called chitolectins) which lack chitinase activity as a result of mutations in their highly conserved putative enzyme sites [56,57]. Among these C/CLPs, Chit1 is the major Cs in humans and the best characterized Cs from a biologic and clinical perspective. In humans, mature monocyte-derived macrophages, Gaucher's cells and lung macrophages express this chitinase. Proinflammatory cytokines such as granulocyte-macrophage colony-stimulating factor, tumor necrosis factor-╬▒, and lipopolysaccharide stimulate the expression of Chit1 in monocyte-derived macrophages, whereas interferon-╬│ and interleukin-4 inhibit Chit1 expression [58,59,60]. Interestingly, Chit1 can be found in detectable quantities in the circulation of normal individuals and is further increased in a variety of diseases characterized by inflammation, tissue remodeling and/or fibrosis including bacterial or fungal infections, lysosomal storage diseases (Gaucher's), sarcoidosis, and interstitial lung diseases [61,62,63,64,65,66]. However, the effector functions of Chit1 have not been clearly defined and its roles in the pathogenesis of specific diseases have not been elucidated. To begin to define the in vivo roles of Chit1 in pulmonary injury and repair, we characterized the levels of circulating Chit1 activity in patients with scleroderma (SSc) and investigated the bleomycin-induced pulmonary responses in newly generated Chit1 null mutant mice (Chit1-/-) and lung-targeted Chit1 overexpressing transgenic mice (Chit1 Tg) [18]. These studies demonstrate that the levels of circulating Chit1 activity are increased in patients with SSc where they correlate with the presence and severity of interstitial lung disease (SSc-ILD). In these studies, the significant role and effect of Chit1 in the pathogenesis of pulmonary fibrosis have been further demonstrated in an animal model of pulmonary fibrosis. The bleomycin-induced pulmonary fibrosis is significantly ameliorated in Chit1-/- mice, but enhanced in Chit1 Tg mice compared to wild type (WT) controls, suggesting that Chit1 plays a critical role in the pathogenesis of pulmonary fibrosis [18].
In earlier studies on SSc-ILD patients, the fibrotic lungs from scleroderma patients showed significant activation (expression) of signature genes associated with TGF-╬▓1 signaling without notable expression of TGF-╬▓1 [67,68]. Interestingly, Tg expression of Chit1 did not increase the expression of TGF-╬▓1 in the lung, however, in a separate in vitro experiment using fibroblast cell line, Chit1 significantly enhanced the TGF-╬▓1-stimulated cellular responses by enhancing TGF-╬▓1 receptor expression as well as canonical and noncanonical signaling pathways of TGF-╬▓1 [18]. These findings led us to speculate that Chit1 contributes to the development of TGF-╬▓1-stimulated pulmonary fibrosis by sensitizing TGF-╬▓1 signaling pathways. If this is the case, Chit1 could be also an effective therapeutic target to block or delay the development or the progression of pulmonary fibrosis in which TGF-╬▓1 plays a significant role.
CONCLUSIVE REMARKS AND FUTURE PROSPECTS
Pulmonary fibrosis is a complex disease with multiple factors are implicated in the development and progression of pathologic tissue responses. As demonstrated in a number of studies, TGF-╬▓1 plays a central role in fibrotic tissue responses including pulmonary fibrosis, and the intervention of TGF-╬▓1 expression or its signaling would be the most promising therapeutic targets for the intervention of fibroproliferative diseases. Accordingly, a number of antibodies, siRNAs and small molecules have been developed to block the expression of TGF-╬▓1 or its receptor or signaling molecules, and some of them are currently under extensive clinical trials [13]. However, because of the vital physiologic function of TGF-╬▓1 in normal immune and cellular homeostasis, direct or complete blocking of TGF-╬▓1 or its signaling would not be tolerable especially in long term therapeutic use. Thus, alternative ways to selectively inhibit the pathologic effect of TGF-╬▓1 while preserving other essential biological function of TGF-╬▓1 would be the best strategy for the treatment of pulmonary fibrosis in which TGF-╬▓1 plays a significant role.
In this regard, recent studies from our laboratory and others identified a number of promising candidate molecules that effectively regulate TGF-╬▓1-stimulated fibrotic tissue responses at different levels, from TGF-╬▓1 activation to extracellular matrix (indicated as A to E in Fig. 1). As discussed above, they are genetically determined modifiers, downstream mediators of TGF-╬▓1, and signaling modifiers. They include integrins (such as integrins av), matrix metalloproteases, semaphorin 7a, phosphatase and tensin homolog agonist, and prostaglandin E2 [69,70,71,72,73]. A number of noncoding microRNAs have been identified to directly or indirectly regulate the expression and signaling of TGF-╬▓1 [11]. The molecules directly associated with collagen processing, such as lysyl oxidase-like 2 [74], could be an effective therapeutic target to reduce the pathologic tissue accumulation of collagen by TGF-╬▓1 stimulation. Since TGF-╬▓1-induced injury responses are crucial for subsequent pulmonary fibrosis, pan-apoptosis inhibitors (such as pan-caspase inhibitors) or mesenchymal stem cells that replace the injured cells could be also therapeutic modifiers of TGF-╬▓1-induced fibrotic tissue responses [75,76]. Although each of these modifiers alone has specific regulatory function on TGF-╬▓1-induced tissue response, targeting multiple modifiers in a patient could provide better therapeutic effect than targeting a single modifier. This needs to be further validated in future studies.
When viewed in combination, a number of "modifiers" of TGF-╬▓1 effector function including AR and Chit1 are already identified, and they are in the main road to be developed as effective and tolerable antifibrotic drugs. Continuous identification and targeting of different types of modifiers of TGF-╬▓1 effector function through systematic and combinatorial approaches will lead us to a better position to control pulmonary fibrosis and other devastating fibrotic diseases in the near future.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





