INTRODUCTION
Ifosfamide is an alkylating agent with remarkable activity against a wide range of tumors. Ifosfamide-induced nephrotoxicity was first described in 1972 and is characterized by glomerulopathy, diabetes insipidus, and proximal tubulopathy [1]. Fanconi syndrome is a rare disease characterized by type II proximal renal tubular dysfunction associated with the urinary loss of phosphate, bicarbonate, potassium, glucose, amino acids, and low-molecular-weight proteins. Ifosfamide-induced Fanconi syndrome develops in children. Only a few cases have been reported in adults, who generally have risk factors such as a cumulative ifosfamide dose > 40-60 g/m2 [2], a reduction in kidney mass [3], or concurrent cisplatin treatment [3]. Diabetes insipidus is another rare complication of ifosfamide-induced renal disease. We herein report a case of ifosfamide-induced Fanconi syndrome with an onset of diabetes insipidus only a few days after the first ifosfamide exposure.
CASE REPORT
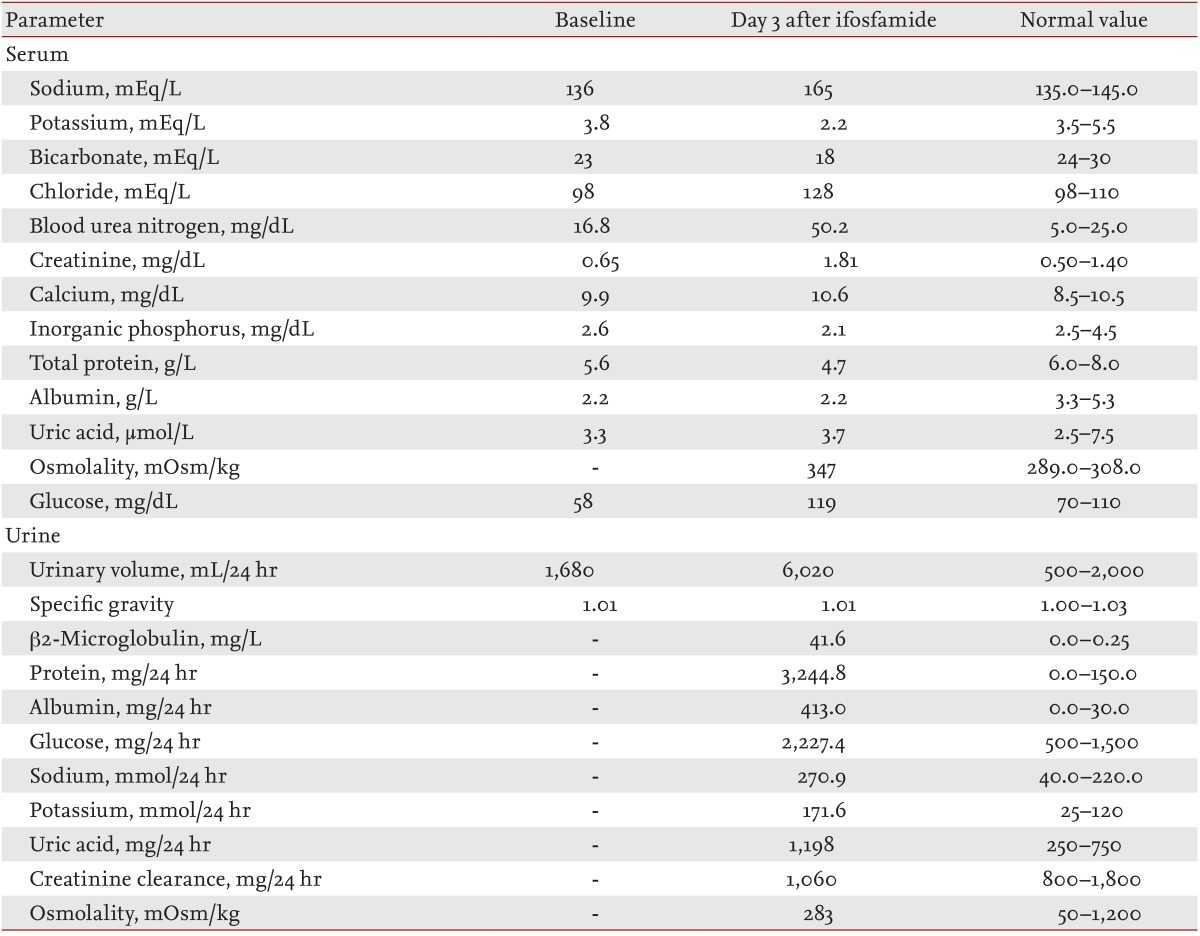
A 61-year-old Russian man was admitted to the clinic for evaluation of a 5-cm axillary mass that had invaded the adjacent bony structures. He was diagnosed with inflammatory malignant fibrous histiocytoma. Neoadjuvant chemotherapy with adriamycin (25 mg/m2) and ifosfamide (2.5 g/m2) was administered on days 1 to 3 following mesna prophylaxis. On day 3 of chemotherapy, the patient showed general weakness, polyuria, and dehydration. Laboratory findings included hypernatremia (serum sodium, 150 mmol/L), hypokalemia (serum potassium, 1.7 mmol/L), hypercalcemia (serum calcium, 10.6 mg/dL), and hypophosphatemia (serum phosphate, 2.1 mg/dL). The combination of hypernatremia, hyperosmolarity, polydipsia, and polyuria were consistent with nephrogenic diabetes insipidus (Table 1). The patient was thus treated with electrolyte replacement and dichlozid. However, his electrolyte imbalances were aggravated by this treatment, and his mental status deteriorated to a stupor. Six days after chemotherapy, his serum blood urea nitrogen and creatinine levels increased to 49.5 mg/dL (normal, 5.0 to 25.0) and 1.81 mg/dL (normal, 0.5 to 1.4), respectively. A 24-hour urinalysis revealed urinary loss of glucose, amino acids, uric acid, and potassium. The presence of these multiple defects in renal proximal tubular reabsorption, including glycosuria, hypophosphatemia, and proteinuria, were suggestive of Fanconi syndrome (Table 1). Treatment of isotonic saline with electrolyte replacement was maintained. However, the electrolyte imbalance was not corrected, and the metabolic acidosis was aggravated by this treatment. Nine days after chemotherapy, the patient died of uncorrected metabolic acidosis.
DISCUSSION
Ifosfamide-induced Fanconi syndrome has been reported in several patients with risk factors such as a cumulative dose > 40-60 g/m2 ifosfamide [2], young age [4], reduction in kidney mass [3], or application of a concurrent nephrotoxic agent such as cisplatin [3]. One case report described a 48-year-old woman with Fanconi syndrome and nephrogenic diabetes insipidus after treatment with a cumulative dose of 41 g/m2 ifosfamide [5]. Her renal function returned to normal after 20 days. Another case report described a 54-year-old man with Fanconi syndrome and nephrogenic diabetes insipidus after ifosfamide treatment (total cumulative dose, 44 g/m2) [6]. Compared with these previous reports, our case is novel in several ways. First, the total dosage of ifosfamide was only 7.5 g/m2 in our patient. Second, our patient had no known risk factors for ifosfamide-induced nephropathy, such as young age, a high total ifosfamide dose, prior or concurrent cisplatin treatment, pre-existing renal impairment, nephrectomy, or tumor infiltration.
The pathophysiology of ifosfamide-induced Fanconi syndrome is unclear. Previous theories have pointed to chloroacetaldehyde as the causal metabolite [7]. Ifosfamide is metabolized into chloroacetaldehyde, which accumulates in the proximal tubular cells and results in nephrotoxicity. In addition, chloroacetaldehyde has been shown to decrease antioxidant glutathione (GSH) and adenosine triphosphate (ATP) levels while inhibiting the activity of NA+/K+-ATPase, which is a V-ATPase. This process is associated with reduced endocytosis and intracellular processing of proteins [8]. Another explanation for the nephrotoxicity is possible depletion of intracellular GSH levels. GSH is the major intracellular scavenger of oxygen radicals and an active metabolite of ifosfamide. 4-Hydroxyifosfamide has been reported to deplete intracellular GSH levels [8].
Renal Fanconi syndrome is a serious condition, and there is no established treatment with the exception of hydration and correction of electrolyte imbalance. Preliminary animal models have shown that the addition of antioxidants, such as N-acetylcysteine, resveratrol, melatonin, L-carnitine, and thymoquinone, to ifosfamide treatment may have promising results, but further research is necessary before these drugs are used as therapeutic options [9,10]. Therefore, early detection of potential tubular dysfunction is necessary. Fanconi syndrome is a disease characterized by type II proximal renal tubular dysfunction consistent with urinary loss of phosphate, bicarbonate, potassium, glucose, amino acids, and low-molecular-weight proteins. Therefore, serum electrolytes, urinary excretion of low-molecular-weight proteins, urinary electrolytes, and renal concentration capacity should be monitored regularly during and after ifosfamide chemotherapy.
In summary, we have reported a case involving a 61-year-old man who developed Fanconi syndrome with nephrogenic diabetes insipidus after ifosfamide chemotherapy. The mechanisms of ifosfamide-induced nephrotoxicity are not clear, and treatments are limited. Close monitoring is needed during ifosfamide treatment.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





