INTRODUCTION
Primary aldosteronism (PA) is a common cause of secondary hypertension, characterized by autonomous aldosterone production [
1]. In hypertensive patients, the prevalence of PA had been considered to be less than 1% [
2,
3] but, recently, it has come to be widely recognized as having a higher prevalence than previously thought. With the widespread use of the aldosterone/renin ratio (ARR) as a screening test, the range has increased, up to 5% to 13%, depending on the population selected and diagnostic values used [
4,
5].
Inappropriately elevated aldosterone acts as a mediator, causing cardiovascular injury via mineralocorticoid receptors (MRs) in the brain, heart, and blood vessels through mechanisms explained by inflammation and endothelial dysfunction [
6]. In 2006, our center conducted a retrospective study [
7] in 41 patients with PA who were diagnosed during the period between 1986 and 2005, and the results showed that they were at increased risk of target organ damage (TOD) compared with patients with essential hypertension. In many studies, it has been documented that appropriate treatment improves high blood pressure (BP) and reverses cardiovascular risks [
8,
9].
Thus, it is important to identify the existence of PA among hypertensive patients. To determine which patients should be suspected for PA and to manage them properly, understanding current trends in PA is helpful. However, few analyses have been conducted to assess the changes in clinical manifestations of PA in Koreans. Here, we retrospectively reviewed chronological changes of the clinical manifestations of aldosteronism since the previous report in 2006.
METHODS
Subjects
We conducted a longitudinal, retrospective study of 85 patients diagnosed with PA at Korea University Anam Hospital over a 27-year period, from January 1986 through March 2012. In total, 41 patients diagnosed between January 1986 and December 2005 were already involved in our previous study, conducted in 2006. Since then, an additional 44 patients were diagnosed with PA between January 2006 and March 2012. Medical records were analyzed retrospectively and the clinical manifestations were compared according to these two time periods.
Screening for and diagnosis of PA
PA was suspected in patients with resistant hypertension, hypertension with hypokalemia (defined as less than 3.5 mmol/L of serum potassium), drug-induced or spontaneous, adrenal incidentaloma, hypertension with a family history of early-onset hypertension, and hypertension diagnosed at a relatively young age. It was screened based on an increased ratio of plasma aldosterone concentration (PAC): plasma renin activity (PRA) ≥ 25 (ng/dL:ng/mL/hr) and the PAC ≥ 15 ng/dL. After this, the diagnosis was confirmed by the lack of aldosterone suppression (≥ 10 ng/dL) after 2 L of intravenous 0.9% saline loading over 4 hours. One subject who had heart failure was confirmed with nonsuppression of plasma aldosterone by the Captopril challenge test to avoid fluid retention, and was considered positive when the ARR ≥ 20. The test was performed under a liberal sodium diet, and serum potassium concentration of less than 3.5 mmol/L was corrected by oral supplementation before assessment. Diuretics, β-blockers, and antagonists of the renin-angiotensin system were discontinued 2 to 4 weeks before assessment.
Subtype evaluation
To differentiate between hyperplasia and adenoma, adrenal computed tomography (CT) scans in all and adrenal venous sampling (AVS) in 17 patients were performed. AVS was conducted by an experienced radiologist. Bilateral sequential catheterization via a percutaneous femoral vein approach was used, and infusion of 50 µg of cosyntropin per hour was initiated 30 minutes before cannulation. AVS was considered successful if plasma cortisol in the adrenal vein was more than 10 times greater than that in the inferior vena cava, which represented the selectivity index. Additionally, the lateralization index, defined as corrected aldosterone/cortisol ratio 4-fold higher than the contralateral side, was indicative of unilateral aldosterone excess. By exclusion, patients with a biochemical diagnosis of PA without evidence for lateralized aldosterone excess were considered to have idiopathic hyperaldosteronism (IHA).
Data collection
Clinical characteristics, including age at diagnosis, gender, body mass index (BMI), smoking, duration of hypertension, history of cerebrovascular or cardiovascular disease and family history of hypertension and cardiovascular disease, were collected from medical records. Baseline BP was obtained from the record of the patients' first visit for evaluation of PA, and posttreatment BP was based on the last follow-up record. Biochemical parameters, including electrolytes, glucose and cholesterol, which were measured simultaneously with PAC and PRA, were obtained. All blood samples were collected with the participants remaining seated for at least 5 to 15 minutes in the morning.
Nephropathy was defined as serum creatinine ≥ 1.5 mg/dL, 24 hour-microalbuminuria ≥ 30 mg/day or proteinuria of ≥ +1 in the urine dipstick test. Retinopathy was considered if fundoscopy showed at least grade I retinopathy according to the Keith-Wagener-Barker classification. To assess the risk of cardiovascular complications, we identified the presence of left ventricular hypertrophy (LVH) on electrocardiography (ECG) or on echocardiogram. In ECG, LVH was diagnosed if the sum of S wave in lead V1 and the R wave in lead V5 or V6, whichever was larger, exceeded 35 mm or the R wave in aVL exceeded 11 mm. Through 2-dimensional guided M-mode echocardiography, left ventricular end-diastolic dimension, interventricular septal thickness, and posterior wall thickness were measured and left ventricular mass (LVM) was calculated using the formula described by Devereux and Reicheck. LVM index (LVMI) was obtained by dividing LV mass by body surface area, and LVH was considered if LVMI was ≥ 134 g/m2 in men and ≥ 110 g/m2 in women.
Statistical analysis
Variables are expressed as means ± SD or ranges, as appropriate. Continuous variables were compared using the t test and categorical variables were compared using the chi-square test and Fisher exact test. Statistical analyses were performed using SPSS software version 18.0 (IBM Co., Armonk, NY, USA). A p value less than 0.05 was considered to indicate statistical significance.
RESULTS
Baseline characteristics
Of the 85 patients, 41 were diagnosed with PA over the 20-year period from January 1986 to December 2005, and the remaining 44 patients were diagnosed over the 6.25-year period from January 2006 to March 2012. The two periods of 1986 to 2005 and 2006 to 2012 showed similarities in the mean age at diagnosis, gender, BMI, and mean duration of hypertension.
In particular, baseline systolic blood pressure (SBP; 184.0 ± 44.8 mmHg vs. 163.7 ± 22.4 mmHg;
p < 0.009) and diastolic blood pressure (DBP; 112.0 ± 23.4 mmHg vs. 100.1 ± 14.9 mmHg;
p < 0.006) were significantly higher in 1986 to 2005, but the subjects were taking fewer antihypertensive agents than patients diagnosed in 2006 to 2012 (1.0 ± 0.8 vs. 1.8 ± 1.3;
p < 0.001). No significant difference was observed in other characteristics, including smoking history and cerebrovascular and cardiovascular diseases. Baseline characteristics are summarized in
Table 1.
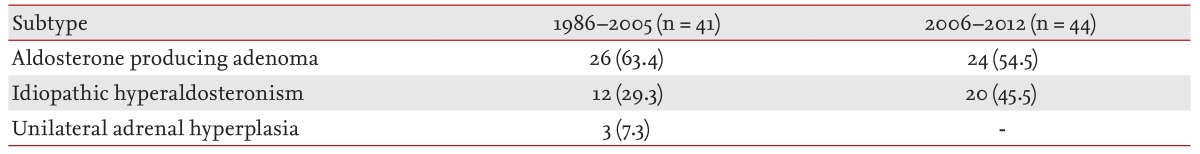
Subtype analysis
Change in subtypes of PA, according to the time period, is summarized in
Table 2. Between 1986 and 2005, aldosterone-producing adenoma (APA) was demonstrated in 26 patients (63.4%) whereas three patients (7.3%) were surgically demonstrated to have unilateral adrenal hyperplasia (UAH), and the remaining 12 patients (29.3%) were diagnosed with IHA. However, during 2006 to 2012, the prevalences of APA and IHA were 24 (54.5%) and 20 (45.5%), respectively. The result showed an increasing tendency in the prevalence of IHA, although it was not statistically significant (χ
2 = 1.65,
p = 0.19).
Biochemical analysis
PAC and PRA were not significantly different between the periods (
Table 3). The patients who were diagnosed in the period 1986 to 2005 showed a mean plasma aldosterone of 39.0 ng/dL and renin activity of 0.37 ng/mL/hr. In 2006 to 2012, the values were 51.1 ng/dL and 0.47 ng/mL/hr, respectively. The ARR was also comparable between the periods (105.4 ± 54.1 [ng/dL:ng/mL/hr] vs. 109.6 ± 69.3 [ng/dL:ng/mL/hr];
p = 0.81).
Of note, in the later period, the mean serum potassium level at the time of diagnosis was significantly higher (2.7 ± 0.6 mmol/L vs. 3.2 ± 0.7 mmol/L; p < 0.0002), and patients who presented with hypokalemia were also fewer in number than in the earlier period (90.2% vs. 61.4%; p < 0.002).
AVS
AVS was performed in 17 patients by an experienced radiologist and no complication was reported. Both adrenal veins were catheterized successfully in all but one patient. Among the 17 patients, 14 cases (82.3%) were examined in the period 2006 to 2012.
Ten patients (62.5%) had a unilateral source of aldosterone hypersecretion. One of the patients showing manifestations of bilateral macronodular lesions on CT turned out to have a unilateral source of aldosterone hypersecretion. The other patient showing the manifestations of unilateral micronodules on CT was found to have a contralateral source of aldosterone hypersecretion. Of patients with IHA, four had unilateral macronodular lesions and one had micronodular lesions on CT. Accordingly, the concordance of CT scan and AVS was 56.2% (
Table 4).
In total, 21 patients younger than 40 years old presented with a high probability of APA because of issues such as high BP, hypokalemia, or unilateral adenoma on CT and thus underwent surgery without AVS. However, in seven of them, the postoperative aldosterone level and BP were not reduced significantly without MR antagonist treatment, and they were finally diagnosed as having IHA.
BP outcome after treatment
Of 50 patients with APA, 45 (90.0%) underwent unilateral adrenalectomy, and the rest of the patients who declined or were unsuitable for surgery were treated with MR antagonists. In contrast, 20 of 32 patients (62.5%) with IHA were initially treated with MR antagonists whereas the other 12 underwent surgery.
We analyzed pretreatment and posttreatment BP values in APA patients who received unilateral adrenalectomy and IHA patients who received medical therapy (
Table 5). APA patients demonstrated higher pretreatment SBP (178.1 ± 29.9 mmHg vs. 163.1 ± 25.7 mmHg;
p = 0.027) and DBP (110.4 ± 19.2 mmHg vs. 100.2 ± 19.6 mmHg;
p = 0.027) than IHA patients. However, after treatment, based on the records of the last follow-up, BP-systolic or diastolic-was comparable between the APA and IHA patients. The reduction in BP was significant in both groups (
p < 0.001). However, there were no differences in BP reduction between the two groups. Additionally, we analyzed the BP outcome in each period. In cases of APA with surgery and IHA with medical treatment, they all showed significant BP reductions after treatment. The changes in BP after treatment in the APA and IHA patients are summarized in
Table 5. In IHA patients who underwent unilateral adrenalectomy, the postoperative BP was not reduced significantly and they needed additional-MR antagonist-treatment.
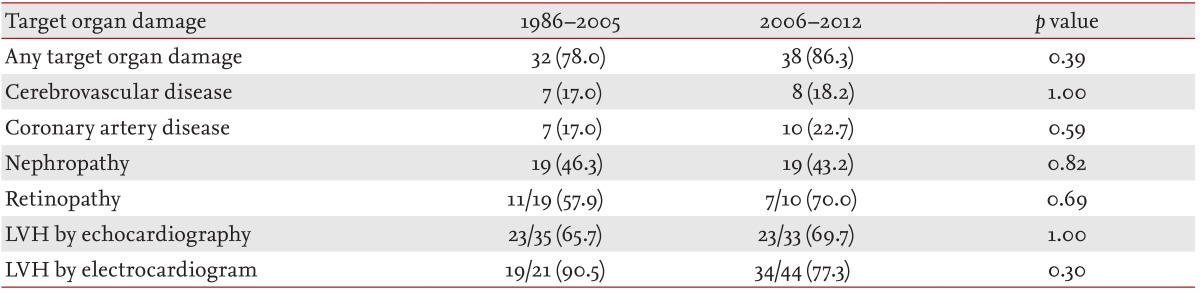
TOD
In the periods 1986 to 2005 and 2006 to 2012, TOD was seen in 32 patients (78.0%) and 38 patients (86.3%), respectively (
p = 0.39). The frequencies of each TOD between the periods of 1986 to 2005 and 2006 to 2012 are summarized in
Table 6. The prevalence of LVH, coronary artery disease, cerebrovascular disease, nephropathy, and retinopathy showed no significant difference between the periods.
Patients with TOD (n = 70, 82.4%), in either period, were relatively old at the time of presentation (patients with TOD vs. without, 46.9 ± 10.5 vs. 39.4 ± 12.1; p = 0.018), had a longer duration of hypertension (6.9 ± 7.1 years vs. 3.8 ± 4.0 years; p = 0.02), and were taking more antihypertensive medications (1.55 ± 1.28 vs. 1.06 ± 0.96; p = 0.10) than patients without TOD (n = 15, 17.6%). However, BP before treatment-systolic (patients with TOD vs. without, 168 ± 34 mmHg vs. 167 ± 27 mmHg; p = 0.94) or diastolic (103 ± 19 mmHg vs. 112 ± 17 mmHg; p = 0.10)-did not differ between the groups.
DISCUSSION
PA was first described by Conn [
10] in 1955, and was characterized by hypertension, hypokalemia, and autonomous production of aldosterone, followed by suppressed renin activity. PA was previously considered to account for less than 1% of hypertensive patients [
2,
3] and hypokalemia was a determinant for pursuing diagnostic tests. However, it is now widely accepted that most patients do not present with hypokalemia, and the popular use of ARR by a simple blood test increased the detection of PA, up to 5% to 13% of all hypertensive patients [
4,
5]. Indeed, Young [
11] reported a 10-fold increase in the prevalence of PA since the introduction of the ARR. Likewise, the detection of PA has increased in our clinic because the numbers of PA patients detected in 1986 to 2005 and 2006 to 2012 were similar. This was the result of increased investigations in normokalemic hypertensive patients, wide application of ARR, and the increased chance of discovering adrenal masses incidentally in CT scans.
In the past, several studies assumed hypokalemia was a hallmark for the diagnosis of PA but recent evidence has challenged this. Gordon [
12] explained that normokalemic PA had not been recognized because it was not considered in screening; however, it clearly existed and its prevalence was simply underestimated. It has also been suggested that it is only valuable to screen for PA in hypokalemic patients based on the belief that most patients with normokalemic PA have IHA rather than APA and are almost surgically incurable [
13]. According to our data, of the 41 patients with PA who were diagnosed during the period between 1986 and 2005 [
7], the results showed that hypokalemia was predominant in APA as a common presentation. However, notably, we also observed that, in the later period, the patients presenting with hypokalemia were fewer in number, with an increasing trend towards IHA. This shift may be explained by earlier and more precise case detection. The hormonal and biochemical abnormalities are generally much milder in patients with IHA than in those with APA [
14] and hypokalemia is usually considered to be more common in APA, especially in severe cases [
15].
On the other hand, Rossi [
16] stated that IHA is increasing because of the more general use of a sensitive screening test, and also by the lack of systematic use of AVS. In a large multicenter study [
17], APAs were detected significantly more commonly in centers with AVS than without. Gordon et al. [
18] were concerned that the prevalence of IHA could have been overestimated in centers not performing AVS, because of the low sensitivity of adrenal CT alone for the detection of small APAs. According to our data, more than half of patients with AVS were diagnosed with APA. Thus, we can expect that AVS may contribute to better estimating the true prevalence of PA subtypes.
AVS was first introduced in 1957 to differentiate the subtype of PA [
19]. It is the most reliable method to date, although the criteria that indicate successful cannulation and lateralization remain controversial. In our study, most AVS (82.3%) were conducted in the later period. On adrenal CT, we observed several inconsistent findings-compared with AVS-and the concordance of CT and AVS was only 56.2%. In another report [
19] of 203 PA patients, CT was accurate in 53% of patients, similar to our data. Consequently, AVS is essential in distinguishing the subtypes of PA; CT alone appears to be an inadequate diagnostic method. Nevertheless, CT should be performed in all patients with suspected PA to exclude large masses that may represent adrenal carcinomas [
15].
In young patients the question of whether to perform AVS is controversial, because it is known that adrenal incidentalomas are uncommon in young patients [
20]. In our study, 21 patients under 40 years old at the time of diagnosis underwent surgery without AVS because the CT scan and other clinical features, such as severe hypertension, spontaneous hypokalemia, and higher levels of aldosterone, were suspicious for APA. However, seven of them were finally diagnosed as having IHA and also needed MR antagonists for BP control. Thus, we can suggest that AVS has clinical importance in patients with PA, regardless of age, especially in those who require surgery.
Unilateral adrenalectomy is effective in patients with APA or UAH to normalize the hypokalemia and to improve BP, whereas medical therapy is the treatment of choice in patients with IHA in whom surgery rarely improves BP [
15]. Thus, distinguishing the subtype of PA is important in assessing treatment plans [
21,
22] and AVS is the most accurate method to determine the etiology of PA.
We analyzed the BP outcome in APA patients treated with adrenalectomy and IHA patients treated with medical therapy. After treatment, the mean BP declined significantly in APA with adrenalectomy and IHA with medical therapy. According to the American Heart Association criteria [
23], cure is defined as SBP < 140 mmHg and DBP < 90 mmHg without antihypertensive medications; and improvement is defined by either SBP < 140 mmHg or DBP < 90 mmHg or both, for patients remaining on the same or fewer medications. Cure and improvement are both considered to have benefit in BP. In our study population, cure was obtained in 53% of APA and in all UAH patients, and improvement was observed in 34% of APA and 87% of IHA patients, respectively. The benefit in BP was similar between APA and IHA. Particularly, among APA patients, all lateralized subjects-by AVS-benefitted from BP reduction after adrenalectomy. Additionally, in all UAH that was confirmed by pathology, BP was cured after adrenalectomy.
In several studies, it has been documented that PA patients are at an increased risk of TOD versus essential hypertensive patients, independent of BP [
7,
24,
25]. We found that about 78.0% versus 86.3% of patients had at least one TOD at the time of diagnosis in the 1986 to 2005 and 2006 to 2012 periods, respectively. None of the values regarding TOD, including LVH, retinopathy, nephropathy, coronary artery disease, and cerebrovascular disease, showed a significant difference over the periods. There was a significant difference when comparing the subgroups between patients with and without TOD. Patients with TOD were much older and had a longer duration of hypertension. Thus, in the case of younger patients with hypertension, patients with adrenal masses, severe hypertension, or a family history of early-onset hypertension, suspicion and screening are important to prevent the occurrence of TOD in PA.
This study has some limitations. First, the study population was small; thus, there was no statistically significant difference in the prevalence of subtypes of PA. Second, because it was a retrospective study, there was a lack of unique criteria for screening PA to compare the precise prevalence. Third, AVS was almost entirely performed in the later period, so the subtypes in the earlier period might not be accurate. Despite these limitations, our study is worthwhile because it is the first conducted in Korea that compares changes in the clinical manifestations of PA over a long period.
In summary, the prevalence of PA is increasing and there is an increasing tendency to IHA. Additionally, hypokalemia is no longer a requisite for the diagnosis of PA. The identification of PA has substantial prognostic value because, after optimal treatment with surgery or medical treatment, BP declines significantly and, furthermore, the increased risk of TOD caused by excess aldosterone can be reduced significantly. Moreover, AVS plays a critical role in distinguishing the subtype of PA to guide proper management, especially in patients who need surgery. With the knowledge that PA is a potentially curable and treatable disease, extending screening, based on current trends, would result in better prognoses.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


