 |
 |
| Korean J Intern Med > Volume 28(4); 2013 > Article |
|
To the Editor,
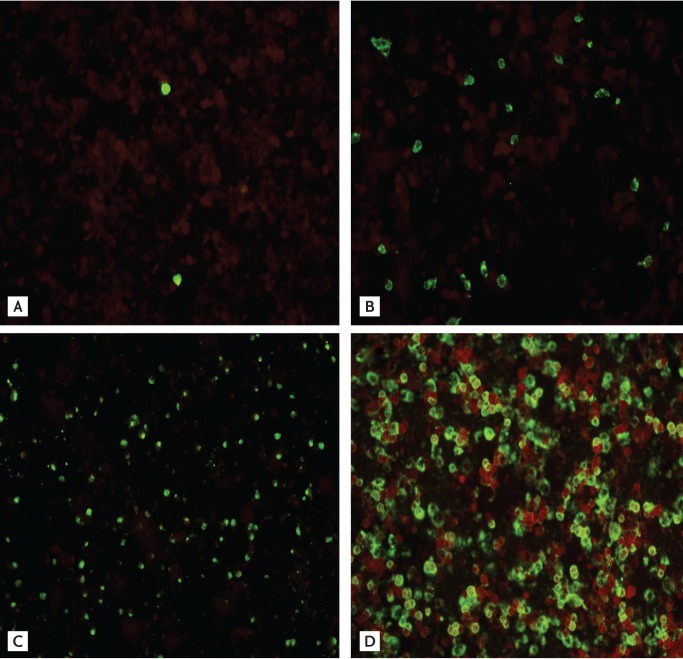
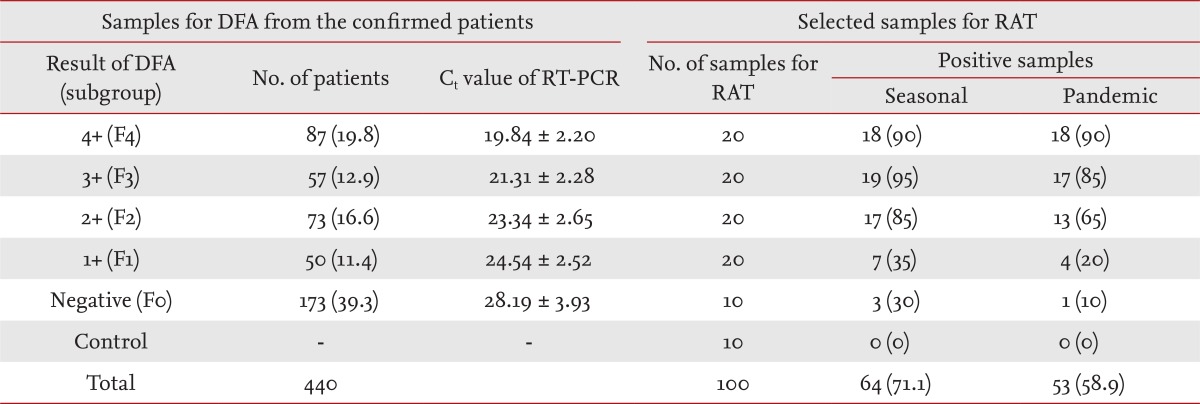
The rapid antigen test (RAT) is a point of care test that provides results within 30 minutes, regardless of the facility. Use of the RAT reduces unnecessary diagnostic testing, facilitates antiviral treatment, and decreases inappropriate use of antibiotics. However, the poor sensitivity of the RAT for influenza virus is a major concern [1]. Lee et al. [2] revealed sample collection time to be an important factor in the accuracy of the RAT. During the 2009 pandemic influenza outbreaks, the seasonal RAT kit, which had been developed for the detection of seasonal influenza virus infections circulating before the 2009 pandemic influenza [3], was used. The pandemic RAT kit was specifically developed for the A (H1N1) pdm09 virus [2]. In our study, we compared the sensitivities of the two RAT kits according to the level of immunofluorescence. From August 2009 to October 2009, samples from 1,366 patients were analyzed concurrently in polymerase chain reaction (PCR) and direct immunofluorescence assay (DFA) tests for detection of the H1N1 virus. Among this cohort, 440 patients were diagnosed with influenza A (H1N1) infection. The samples were collected using flocked nasopharyngeal swab, and used in the PCR and DFA tests within 12 hours of collection. The remaining samples were stored frozen at -80Ōäā until use. Retrospectively, we used the two RAT kits to test 90 randomly selected clinical samples from 90 patients. In addition, 10 control samples were taken from H1N1-negative patients. The 2009 H1N1 influenza diagnosis was confirmed by real-time reverse transcription (RT)-PCR with a cycle threshold (Ct) of 37. All PCR testing for H1N1 infection was conducted according to the protocol of the Korea Centers for Disease Control and Prevention. D3 Respiratory Virus Reagents (Diagnostic Hybrids, Athens, OH, USA) were used for the DFA tests. Each well was examined under a microscope (magnification, ├Ś 100 to ├Ś 200) for the presence of two or more intact cells with a specific fluorescence pattern. The level of fluorescence was recorded, as follows, according to the number of positive cells: 4+, positive cells distributed compactly and evenly and forming aggregates; 3+, positive cells distributed evenly but not compactly; 2+, easily detectable positive cells scattered loosely; 1+, indistinct positive cells scattered very loosely (Fig. 1). The RATs were conducted with the seasonal SD Bioline Influenza A/B kit (Standard Diagnostics, Yongin, Korea) and the pandemic SD Bioline Influenza A/B/A (H1N1) kit (Standard Diagnostics). Samples were classified into five subgroups according to the DFA results: negative (F0); 1+ (F1); 2+ (F2); 3+ (F3); and 4+ (F4). We calculated the sensitivities and specificities of the DFA and the RATs and compared the sensitivities of the two RATs. The sensitivities of the seasonal RAT and pandemic RAT were 71.1% (64/90) and 58.9% (53/90), respectively. There was a noticeable difference in RAT sensitivity between the subgroups (Table 1). RAT sensitivities were markedly higher in F3 and F4 than in F1 and F0, in which the sensitivity of pandemic RAT was < 20%. The specificities of the seasonal RAT and pandemic RAT were 100%. In our study, both the seasonal RAT and pandemic RAT showed moderate levels of sensitivity (71.1% and 58.9%, respectively). Unexpectedly, the sensitivity of the pandemic RAT was lower than that of the seasonal RAT. In a previous study that used titer pandemic influenza A/H1N1 virus grown in cell culture, the viral loads required to obtain positive results were 1.0 to 1.5 logs higher than those required in the case of seasonal virus [4]. Thus, manufacturers need to improve the sensitivity of the pandemic RAT kit. The concentrations of viruses in clinical samples influence the outcome of the RAT [5]. In this study, the sensitivity of the RAT varied according to the level of fluorescence. Higher levels of fluorescence corresponded to lower mean Ct values in the RT-PCR and markedly increased sensitivity of the RAT. This finding is consistent with previously published results [1,2,5]. Our findings indicate that the sensitivity of RAT varies according to the level of fluorescence, regardless of the RAT kit used.
Acknowledgments
Some of the data were presented at the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, USA, September 9 to 12, 2011. This study was supported in part by the Foundation of Wonkwang University in 2013.
References
1. Vasoo S, Stevens J, Singh K. Rapid antigen tests for diagnosis of pandemic (Swine) influenza A/H1N1. Clin Infect Dis 2009;49:1090ŌĆō1093PMID : 19725784.


2. Lee CS, Lee JH, Kim CH. Time-dependent sensitivity of a rapid antigen test in patients with 2009 H1N1 influenza. J Clin Microbiol 2011;49:1702. PMID : 21248095.



3. Choi WS, Noh JY, Huh JY, et al. The clinical usefulness of the SD Bioline Influenza Antigen Test(R) for detecting the 2009 influenza A (H1N1) virus. Yonsei Med J 2011;52:683ŌĆō685PMID : 21623614.



Figure┬Ā1
Levels of fluorescence according to the numbers of positive cells. (A) F1, indistinct positive cells scattered very loosely. (B) F2, easily detectable positive cells scattered loosely. (C) F3, positive cells distributed evenly but not compactly. (D) F4, positive cells distributed compactly and evenly and forming aggregates.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



