 |
 |
| Korean J Intern Med > Volume 27(4); 2012 > Article |
|
Abstract
Background/Aims
Spontaneous reporting systems have several weak points, such as low reporting rates and insufficient clinical information. Active surveillance programs, such as ward rounds and a clinical data repository (CDR), may supplement the weak points of such systems. We developed active surveillance programs and compared them with existing spontaneous reporting.
Methods
We collected adverse drug event (ADE) cases, which comprised 1,055 cases of spontaneous reporting, 309 reported by ward rounds, and 229 found using a CDR. The clinical features and causative drugs were evaluated.
Results
Active surveillance programs detected additional serious ADEs compared to those of spontaneous reporting programs. The ADEs identified by CDR (22.9%) were more likely to be classified as "serious" than those reported spontaneously (5.2%) or identified during ward rounds (10.3%). Causative drugs also differed. Opioids, antibiotics, and contrast media were the most common drugs causing ADEs in the spontaneous reporting system, whereas the active surveillance programs identified antibiotics as the most common causative drug. Clinical features also differed. ADEs with gastrointestinal manifestations were reported most frequently by spontaneous reporting programs. ADEs reported from active surveillance more reliably identified events associated with changes in laboratory values, such as hepatobiliary toxicity, hematologic manifestations, and nephrologic manifestations, compared with spontaneous reporting programs.
Adverse drug events (ADEs) are the most common safety-concerning events occurring in hospitals [1-3]. ADEs occur in 2.4 to 5.2/100 adult hospitalized patients [4-7], increasing both the length of hospital stays by 2.2 days and hospital costs by $1,900 to $5,900 [4,8]. Recent studies have shown that 44% to 78% of ADEs may be due to systemic problems that could be corrected by an adequate information system [9,10].
Two different methods of collecting ADE reports are available [11]. One is traditional ADE reporting, which is called "spontaneous reporting." The other is "active surveillance," using a phone-structured interview [12], ward rounds and chart review [13,14] or computer monitoring [15-17]. Traditional, spontaneous reporting systems have long been the primary mechanism by which institutions identify ADEs [14,18-21], but they have proven ineffective due to under-reporting. Spontaneous reporting identifies at most only 5% of all ADEs [22]. Besides under-reporting problems, clinical information from spontaneously reported ADEs sometimes lacks essential data, such as a lack of temporal relationship, responses to challenges and/or re-challenge, and underlying patient condition, each of which are essential for identification of the causality of suspected drugs.
Various active surveillance programs are used for pharmacovigilance in hospitals. 'Ward rounds and chart review' examines ADEs documented in the chart [13,16]. Ward rounds and chart review is a prospective method that reveals detailed information about an ADE, but the burden of cost is substantial, and it requires training chart reviewers as to the definitions of ADEs and which triggers to look for. Computer monitoring is also used to screen both administrative and clinical databases for ADEs, based on a set of rules [7,21]. When a suspected event is flagged by the system, a pharmacist or trained investigator performs a targeted chart review to verify the event. This is much less labor-intensive than a routine chart review. Many hospitals, including ours, have recently replaced paper charts with electronic medical record (EMR) systems, permitting easy identification of suspected ADEs from the databases of either in- or outpatients.
Severance Hospital recently developed an active surveillance pharmacovigilance program that involves ward rounds followed by chart review and the inpatient clinical database, in addition to a traditional spontaneous reporting program. In this study, we retrospectively evaluated the performance of these active and spontaneous reporting programs and found significant differences in the clinical features of the ADEs collected by these programs.
A total of 1,593 adverse drug reactions (ADRs) were collected from Severance Hospital, which is a 2,100-bed, tertiary-care general teaching hospital located in Seoul, from October 2009 to July 2010. The hospital has adopted an EMR system. This system was developed by LG CNS (Seoul, Korea) and contains comprehensive data, including patient demographic information, prescriptions, diagnostic codes, laboratory results, medication profiles, vital signs, progress notes by doctors, and nursing notes. Three methods of collecting ADEs are used at Severance Hospital. A spontaneous reporting system relies on electronic reporting by nurses, pharmacists, and doctors. We educate and encourage these medical professionals to report any suspected ADEs. A second pharmacovigilance method uses ward rounds and a chart review by doctors, pharmacists, and specialized nurses. Of the 68 wards in our hospital, we perform ward rounds in only 30 due to limited resources. The inspectors ask nurses or residents at the wards for the suspected ADE cases, and the patient electronic records are then reviewed. The third method is an analysis of the clinical data repository (CDR). Since December 2007, Severance Hospital has used the Clinical Data Research System (CDRS; LG CNS), an internally developed hospital information system that collects comprehensive data, including orders, diagnostic codes, patient demographic information, laboratory results, medication profiles, nursing notes, and vital signs. We identified ADEs using the CDRS at 2-week intervals beginning in April 2010. A trained reviewer performed a targeted review of each patient's electronic medical chart, which was then associated with a specific ADE. We monitored five terms, "drug," "adverse effect," "poisoning," "intoxication," and "toxicity," listed in either the final diagnosis of the discharge summary or on consultation forms during admission. The EMR charts of suspected CDR cases were reviewed by a trained physician who then reported the cases to the pharmacovigilance system as hospital ADEs. All ADE reports from spontaneous and active surveillance programs were verified by the ADR Monitoring Committee of Severance Hospital. The identified ADEs were characterized in terms of seriousness, causality, type, and associated clinical manifestations. We used the World Health Organization (WHO) Adverse Reaction Terminology to report the ADRs. This terminology is hierarchical, and consists of four terms: a "systemic organ" term, broad "high-level" terms, a specific and disease-related or symptom-related "preferred" term, and the frequently reported alternative "included" term [23]. We assessed causality using criteria from the WHO Uppsala Monitoring Center (UMC), which consists of six levels of global introspection, as follows: certain, probable/likely, possible, unlikely, conditional/unclassified, and inaccessible/unclassifiable [24]. ADE severity was categorized as either serious or non-serious. The serious category included life-threatening events, death, permanent disabilities, congenital abnormalities, or prolonged hospitalization. ADRs were further classified by reaction type [24]. Type A reactions were usually dose-dependent, predictable, preventable, and of low mortality, whereas type B reactions were not dose-dependent but were idiosyncratic, unpreventable, and resulted in high mortality. Type C reactions were both dose- and time-related.
Descriptive statistics were used to assess the ADE cases. Results are reported as means and standard errors, except for demographic data. ADE rates among the ADE monitoring programs were compared using the chi-square test for goodness of fit. All calculations were performed using the SAS version 9.1.3 (SAS Institute, Cary, NC, USA).
We collected 1,593 ADE cases. Of these, 1,055 (66.2%) were obtained by spontaneous reporting, 309 (19.4%) by ward rounds, and 229 (14.4%) by CDR.
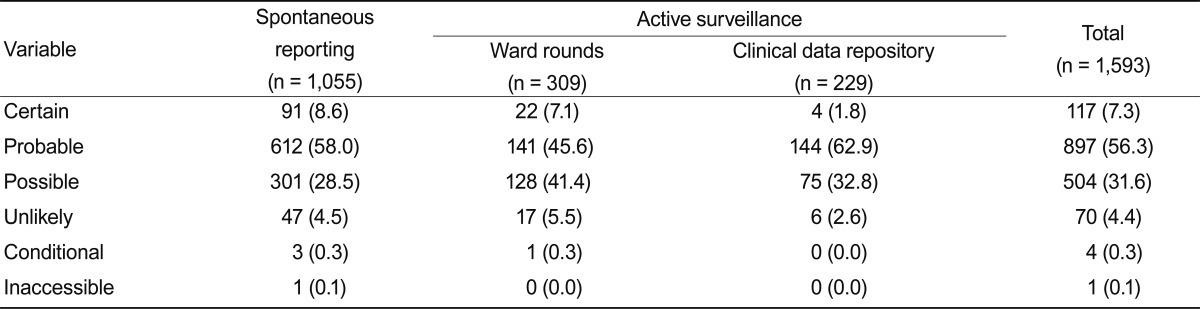
Median patient age was 51.3 years, and females (52.5%) predominated over males (47.5%). Causality assessments for spontaneously reported and active surveillance ADEs were not significantly different. Probable causality (56.3%) was the most common assessment, followed by possible (31.6%), certain causality (7.3%), unlikely (4.4%), conditional (0.3%), and inaccessible (0.1%) (Table 1). Nurses reported using spontaneous reporting more frequently (73.8%) than did doctors (24.8%).
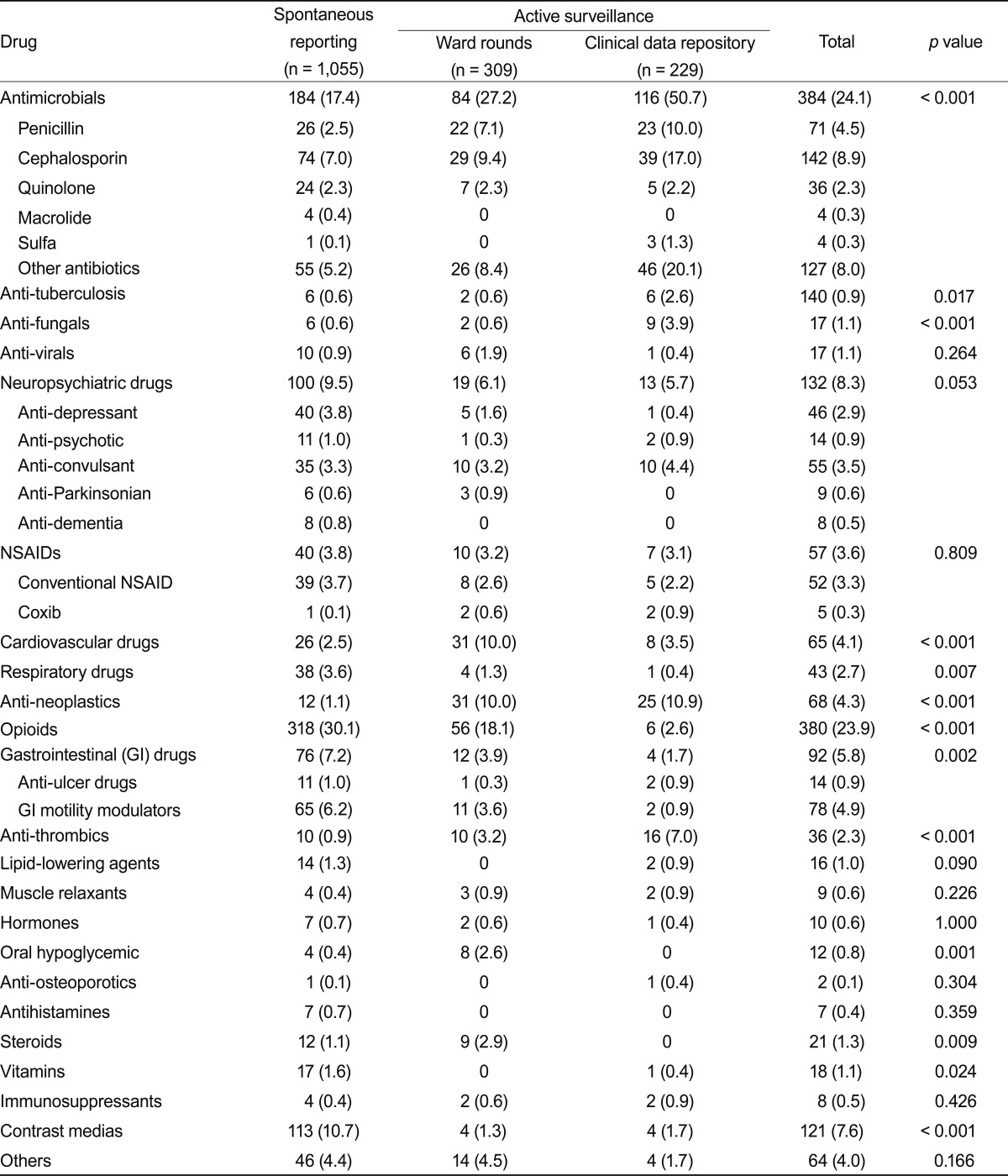
The causative drugs in this study are shown in Table 2. Opioids (30.1%) were the most common causative drug in the spontaneous reporting program, followed by antibiotics (17.4%), contrast media (10.7%), and neuropsychiatric drugs (9.5%). Antibiotics (37.2%) were the most common causative drugs associated with active surveillance, followed by opioids (11.5%), anti-neoplastic agents (10.4%), and cardiovascular agents (7.3%). Cephalosporins (8.9%) were the most frequently identified antibiotics.
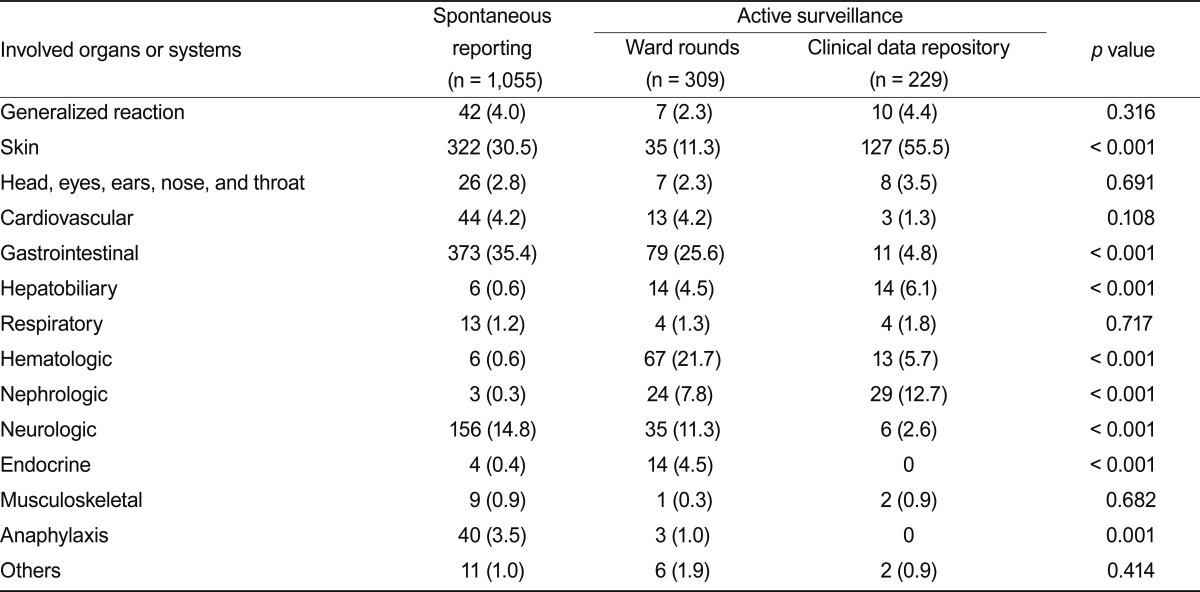
The CDR identified serious ADEs more frequently than did the other methods. The CDR detected 52 serious ADEs of a total of 229 (22.7%), compared to 55 serious ADEs of 1,055 reported spontaneously (5.2%), and 32 serious ADEs of 309 reported by ward rounds (10.4%) (Fig. 1A). Most ADRs identified by CDR were type B reactions (69.9%), whereas 36.0% of spontaneously reported ADEs and 27.8% of those identified by ward rounds and chart review were classified as type B (Fig. 1B). Of the type B reactions identified by the CDR, 24.6% were considered serious. Gastrointestinal involvement was the most frequent clinical manifestation identified by the spontaneous reporting program, occurring in 35.4%, followed by skin involvement (30.5%) and neurologic involvement (14.8%), including headache, dizziness, and insomnia (Table 3). The active surveillance program detected skin involvement frequently (30.1%). However, the active surveillance program also detected ADEs associated with laboratory abnormalities, such as hematologic manifestations (14.9% vs. 0.6%, p < 0.001), hepatobiliary toxicity (5.2% vs. 0.6%, p < 0.001), and nephrotoxicity (9.9% vs. 0.3%, p < 0.001), more reliably than did either of the other systems (Fig. 1C).
Spontaneous reporting facilitates identification of possible ADEs and drug associations but has a number of limitations. Under-reporting is a common problem with spontaneous surveillance [25], and analysis of passive surveillance data does not yield an incidence rate. Thus, multidisciplinary programs are required to detect ADEs in a hospital [14]. Active surveillance can be defined as the periodic collection of case reports from healthcare data systems. Active surveillance systems, such as ward rounds associated with chart review and the CDR, increase the rate of ADRs detection in a hospital setting. Most reporters using the spontaneous reporting program in this study were nurses. Motivating doctors to report ADEs spontaneously is difficult [20], and nurse and pharmacist participation may be essential for successful implementation of a spontaneous pharmacovigilance program in a hospital [14,26,27]. However, nurses and pharmacists may not be aware of all aspects of the ADEs occurring in a hospital, so additional active programs may be needed. In this study, we found little overlap in the cases reported by the spontaneous and active surveillance programs, which might reflect the different characteristics of the ADEs collected. The most common cause of spontaneously reported ADEs was opioids. Monitoring pain control is an important duty of nurses, and patients frequently complain of gastrointestinal symptoms. This result may reflect the characteristics of the patients admitted to our hospital. In Korea, bipolarization of the use of medical services is becoming a serious problem. Patients who require anti-cancer chemotherapy, invasive medical treatment, or surgical intervention are becoming more favored in major general hospitals in Seoul, including ours. As a consequence, use of opioids is relatively more frequent than at other general hospitals in Korea.
Antibiotics were the most common causative drugs identified by both ward rounds and the CDR, as has been reported previously. For example, Hunziker et al. [28] reported that the most common drugs causative of cutaneous drug reactions were penicillin antibiotics, cotrimoxazole, and non-steroidal anti-inflammatory drugs, using a computerized database between 1974 and 1993. However, the proportions of ADRs due to quinolone and vancomycin are high. The high frequency of ADRs due to vancomycin may be because to 75% of the data originated in the six general hospitals that care for the most serious patients, and the relatively high prevalence of methicillin-resistant Staphylococcus aureus in Korea [29].
We found that the active surveillance program effectively detected additional serious ADEs and those associated with laboratory abnormalities. A significant number of additional type B reactions were identified by the CDR. This may have been attributable to the signals used for CDR monitoring, which include toxicity, poisoning, and intoxication; these might be associated with ADE seriousness or those related to laboratory anomalies. Serious ADEs constitute a major public health concern as causes of morbidity, mortality, and high medical expense, reinforcing the importance of implementation of active surveillance systems for patient safety in hospitals. Similar results have been reported previously [15,17]. Jha et al. [15] reported that active surveillance models, such as chart review and computer monitoring, detected more serious ADEs or those associated with laboratory values, such as renal failure or hypoglycemia, more effectively than did other methods. Although our and their results were similar, the monitoring rules were quite different. They used 52 rules, which were modified from the LDS Hospital Study rules [30]; those can be classified into orders for known antidotes, laboratory abnormalities, and laboratory abnormalities occurring in the presence of certain drugs. We used simple and intuitive rules, such as the drug, adverse effect, poisoning, intoxication, or toxicity in the final diagnosis of the discharge summary or the consultation form. Further studies may be needed to verify the appropriateness of our rules for detection of additional ADEs in other hospitals.
While we found differences among the three reporting models, they each had their limitations. A spontaneous reporting program requires health professionals to decide which drug caused the event, describe the event, and determine what actions to take to correct the problem. Health professionals are usually not well-motivated and may not know the significance of post-market surveillance included in a spontaneous pharmacovigilance program [20]. Health professionals must be educated on the importance of spontaneous ADE reporting. The CDR had several advantages compared to a spontaneous reporting system, including cost-effectiveness [15] and is less dependent on the education and motivation of health professionals. We recruited one additional nurse for ward rounds, but we did not recruit any additional employees for the CDR. However, our CDR program had several limitations. Because we reviewed the CDR data retrospectively, we were unable to obtain additional information by interviewing the patients or information providers. We monitored the final diagnosis for five signals in the discharge summary and the consultation form, but doctors may not have described insignificant ADEs or may have deleted the description entirely [15]. Therefore, this method may also be subject to under-reporting. Lag time between ADE occurrence and registration after causality assessment was also inevitably significant with our CDR method, and most of the patients with signs were discharged at the time of the causality assessment. Using the ward round and chart review system, we could interview patients and obtain high-quality data, but this is costly and labor-intensive, and conducting ward rounds in all wards may not be practical [15]. Thus we included rounds in only 30 of 68 wards, which might have caused selection bias, and so the data might not be representative of the general features of ADEs at this hospital.
The ultimate goal of a hospital pharmacovigilance program is prevention of ADRs. Studies have shown that 19% to 61% of ADRs that occur in a hospital are preventable [31-33]. We registered reported ADEs to the EMR system if they belonged to the certain, probable, or possible classes of the WHO UMC causality-assessment criteria, and the referee of the Drug Adverse Event Monitoring Committee of our hospital judged that the reported ADEs were clinically significant. Then, if a physician prescribed a similar class of drug to that which resulted in a registered ADE, a pop-up window was generated in the EMR to remind the physician that the patient had experienced an ADE. Furthermore, the Medication Management Use Committee of our hospital reviewed frequent elicitors of ADEs and created a program to prevent ADE occurrence, which included dropping out frequent elicitors from the available drug list and registering drugs that frequently elicited serious ADEs as high-alert medications. In addition, non-preventable ADEs could in future become preventable through use of new technologies to administer hospital services.
We conclude that active surveillance programs detect more serious ADEs and ADEs related to laboratory abnormalities than did either of the other programs. Spontaneous pharmacovigilance systems are insufficient; thus, active surveillance programs should be implemented in the hospital setting to ensure patient safety.
Acknowledgments
This research was supported by a grant (07072KFDA213) from the Korea Food & Drug Administration.
References
1. Forster AJ, Halil RB, Tierney MG. Pharmacist surveillance of adverse drug events. Am J Health Syst Pharm 2004;61:1466ŌĆō1472PMID : 15332694.


2. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients: results of the Harvard Medical Practice Study II. N Engl J Med 1991;324:377ŌĆō384PMID : 1824793.


3. Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care 2000;38:261ŌĆō271PMID : 10718351.


4. Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients: excess length of stay, extra costs, and attributable mortality. JAMA 1997;277:301ŌĆō306PMID : 9002492.


5. Senst BL, Achusim LE, Genest RP, et al. Practical approach to determining costs and frequency of adverse drug events in a health care network. Am J Health Syst Pharm 2001;58:1126ŌĆō1132PMID : 11449856.


6. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention: ADE Prevention Study Group. JAMA 1995;274:29ŌĆō34PMID : 7791255.


7. Nebeker JR, Hoffman JM, Weir CR, Bennett CL, Hurdle JF. High rates of adverse drug events in a highly computerized hospital. Arch Intern Med 2005;165:1111ŌĆō1116PMID : 15911723.


8. Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients: Adverse Drug Events Prevention Study Group. JAMA 1997;277:307ŌĆō311PMID : 9002493.


9. Leape LL, Bates DW, Cullen DJ, et al. Systems analysis of adverse drug events: ADE Prevention Study Group. JAMA 1995;274:35ŌĆō43PMID : 7791256.


10. Raschke RA, Gollihare B, Wunderlich TA, et al. A computer alert system to prevent injury from adverse drug events: development and evaluation in a community teaching hospital. JAMA 1998;280:1317ŌĆō1320PMID : 9794309.


11. Weaver J, Willy M, Avigan M. Informatic tools and approaches in postmarketing pharmacovigilance used by FDA. AAPS J 2008;10:35ŌĆō41PMID : 18446503.



12. Zancan A, Locatelli C, Ramella F, et al. A new model of pharmacovigilance? A pilot study. Biomed Pharmacother 2009;63:451ŌĆō455PMID : 18790597.


13. Bates DW, Leape LL, Petrycki S. Incidence and preventability of adverse drug events in hospitalized adults. J Gen Intern Med 1993;8:289ŌĆō294PMID : 8320571.


14. Keith MR, Bellanger-McCleery RA, Fuchs JE Jr. Multidisciplinary program for detecting and evaluating adverse drug reactions. Am J Hosp Pharm 1989;46:1809ŌĆō1812PMID : 2801715.


15. Jha AK, Kuperman GJ, Teich JM, et al. Identifying adverse drug events: development of a computer-based monitor and comparison with chart review and stimulated voluntary report. J Am Med Inform Assoc 1998;5:305ŌĆō314PMID : 9609500.



16. Gandhi TK, Seger DL, Bates DW. Identifying drug safety issues: from research to practice. Int J Qual Health Care 2000;12:69ŌĆō76PMID : 10733086.


17. Hwang SH, Lee S, Koo HK, Kim Y. Evaluation of a computer-based adverse-drug-event monitor. Am J Health Syst Pharm 2008;65:2265ŌĆō2272PMID : 19020194.


18. Faich GA. National adverse drug reaction reporting: 1984-1989. Arch Intern Med 1991;151:1645ŌĆō1647PMID : 1872669.


19. Berry LL, Segal R, Sherrin TP, Fudge KA. Sensitivity and specificity of three methods of detecting adverse drug reactions. Am J Hosp Pharm 1988;45:1534ŌĆō1539PMID : 3046347.


20. Rogers AS, Israel E, Smith CR, et al. Physician knowledge, attitudes, and behavior related to reporting adverse drug events. Arch Intern Med 1988;148:1596ŌĆō1600PMID : 3382304.


21. Schiff GD. Using a computerized discharge summary data base check box for adverse drug reaction monitoring. QRB Qual Rev Bull 1990;16:149ŌĆō155PMID : 2113667.


22. Cullen DJ, Bates DW, Small SD, Cooper JB, Nemeskal AR, Leape LL. The incident reporting system does not detect adverse drug events: a problem for quality improvement. Jt Comm J Qual Improv 1995;21:541ŌĆō548PMID : 8556111.


23. The Uppsala Monitoring Centre. The WHO-ART Adverse Reaction Terminology. 1992;Uppsala: The Uppsala Monitoring Centre.
24. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000;356:1255ŌĆō1259PMID : 11072960.


25. Vallano A, Cereza G, Pedros C, et al. Obstacles and solutions for spontaneous reporting of adverse drug reactions in the hospital. Br J Clin Pharmacol 2005;60:653ŌĆō658PMID : 16305591.



26. Miwa LJ, Randall RJ. Adverse drug reaction program using pharmacist and nurse monitors. Hosp Formul 1986;21:1140ŌĆō1146PMID : 10279322.

27. Marcondes RA. Dental care of the cardiac patient. Ars Curandi Odontol 1976;2:3ŌĆō8PMID : 1072960.

28. Hunziker T, Kunzi UP, Braunschweig S, Zehnder D, Hoigne R. Comprehensive hospital drug monitoring (CHDM): adverse skin reactions, a 20-year survey. Allergy 1997;52:388ŌĆō393PMID : 9188919.


29. Choi JH, Shin YS, Suh CH, Nahm DH, Park HS. The frequency of adverse drug reactions in a tertiary care hostpital in Korea. Korean J Med 2004;67:290ŌĆō296.
30. Classen DC, Pestotnik SL, Evans RS, Burke JP. Computerized surveillance of adverse drug events in hospital patients. JAMA 1991;266:2847ŌĆō2851PMID : 1942452.


31. Kunac DL, Kennedy J, Austin N, Reith D. Incidence, preventability, and impact of Adverse Drug Events (ADEs) and potential ADEs in hospitalized children in New Zealand: a prospective observational cohort study. Paediatr Drugs 2009;11:153ŌĆō160PMID : 19301935.


Figure┬Ā1
(A) Percentages of serious adverse drug events (ADEs) identified by spontaneous reporting and two active surveillance programs. (B) Percentages of type B ADEs identified by spontaneous reporting and two active surveillance programs. (C) Percentages of ADEs associated with laboratory abnormalities identified by spontaneous reporting and two active surveillance programs. Laboratory abnormalities included hepatic, hematologic, and nephrotic ADEs. CDR, clinical data repository. ap < 0.01, bp < 0.05.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



