 |
 |
| Korean J Intern Med > Volume 27(2); 2012 > Article |
|
Abstract
Because Mongolia has much higher liver disease burden than any other regions of the world, it is necessary to provide information on real-time situation of chronic liver disease in Mongolia. In this article, we reviewed studies performed in Mongolia from 2000 to 2011 on seroprevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) among healthy individuals and patients with chronic liver diseases, and on the practice patterns for the management of liver cirrhosis and hepatocellular carcinoma (HCC). According to previous reports, the seroprevalence of HBV and HCV in general population in Mongolia is very high (11.8% and 15% for HBV and HCV, respectively). Liver cirrhosis is also highly prevalent, and mortality from liver cirrhosis remained high for the past decade (about 30 deaths per 100,000 populations per year). Among patients with cirrhosis, 40% and 39% are positive for HBsAg and anti-HCV, respectively, and 20% are positive for both. The seroprevalence is similar for HCC and more than 90% of HCC patients are positive for either HBV or HCV. The incidence of HCC in Mongolia is currently among the highest in the world. The mortality from HCC is also very high (52.2 deaths per 100,000 persons per year in 2010). Partly due to the lack of established surveillance systems, most cases of HCC are diagnosed at an advanced stage. The mortality from liver cirrhosis and HCC in Mongolia may be reduced by implementation of antiviral therapy program and control of alcohol consumption.
Mongolia has the highest incidence of hepatocellular carcinoma (HCC) and liver cirrhosis (LC) worldwide (Fig. 1). HCC occurs in 61.9 patients per 100,000 population each year, and LC occurs in 350 patients per 100,000, and its high occurrence has been attributed to the high prevalence of chronic viral hepatitis [1]. We explain the current situation regarding HCC and LC in Mongolia.
The hepatitis B (HBV) and C viruses (HCV) are highly prevalent in Mongolia. The seroprevalence of HBV is 11.8% in the unvaccinated population (meta-analysis of eight studies) [2], and that of HCV is 15.6% in the apparently healthy population [3].
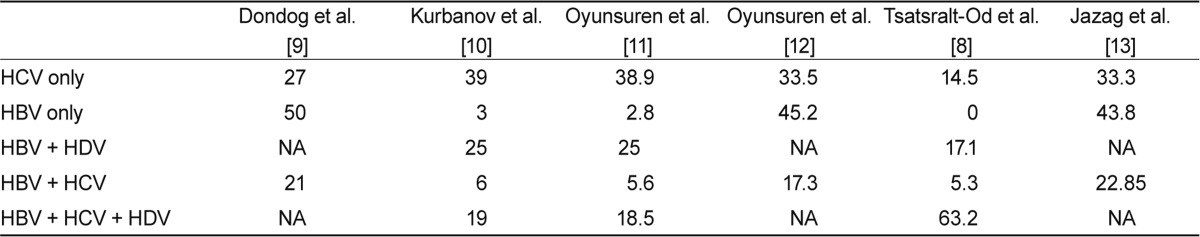
The prevalence of chronic HCV infection in Mongolia is strikingly high considering that the global HCV carrier prevalence is estimated to be about 3%, ranging from 0.1 to 10% or more [4]. Therefore, Mongolia has the highest prevalence (> 15%) of HCV and (> 10%) HBV infection, along with Egypt and Tanzania [5,6]. This high prevalence is attributed to improper sterilization and disinfection of medical and dental equipment that might contribute to the spread of hepatitis viruses [3]. Interestingly, most HCV infections are caused by genotype 1 (98.8%) in patients with chronic hepatitis, and genotype 2 infection is very rare (1.2%) [3]. In this regard, improvements in blood safety as well as the development and execution of strict disinfection and sterilization guidelines for health-related procedures such as phlebotomy, injection, and dental and surgical manipulations are required to control the spread of hepatitis viruses in Mongolia. Besides chronic hepatitis C, Mongolia is confronted with a high prevalence of chronic hepatitis B (Table 1). The HBV surface antigen (HBsAg) prevalence is 9-10% of healthy individuals in Mongolia [7]. With such a high HBV carrier rate in the general population, HBV infection and its associated complications pose serious health problems. To reduce the rate of HBV infection via mother-to-baby transmission during infancy, the Mongolian government launched a universal HBV immunization program in 1991. Most HBV infections are caused by genotype D (88.8%). Genotypes A, B, and C infection are rare at 0.9, 0.9, and 6.0% respectively [8]. One study demonstrated that 10-50% of HBV immunization attempts failed and that HBV vaccine failure is not associated with HBV mutants but is often associated with an inability to simultaneously administer the vaccine and immunoglobulin at birth.
LC is highly prevalent in Mongolia (Table 2). Most LC is attributable to HCV, HBV, and hepatitis D virus (HDV), and a small portion is due to alcohol (Fig. 2). Among patients with cirrhosis, 40% (95% confidence interval [CI], 37 to 43) are HBsAg+; 39% (95% CI, 36 to 42) are antiHCV+, 20% (95% CI, 18 to 23) are dual+, and 1% are negative [9] (Fig. 3). In the last 10 years, cancers have been second among the causes of mortality in Mongolia. According to the 2010 national statistics, HCC is the most common malignancy, representing 44.2% of all cancers in Mongolia (Fig. 4). An increase in the incidence and deaths from HCC has been observed in recent years (Fig. 5). The incidence of HCC is six times higher than the global average (Fig. 6). In Europe, the USA, and Japan, HCV is the predominant cause of development of HCC, whereas HBV infection is the main cause of HCC in Korea. Mortality in males is mainly due to cancers of the liver and stomach and in females it is most commonly due to liver and cervical cancers. Higher incidences of cancer are observed in adults aged 45 to 60 years (Table 3). The 1-year HCC survival rate is 21-24%, that at 3 years is 10-11%, and at 5 years is 6-8% according to an unpublished Natioanal Cancer Center report. No data LC survival are available.
Diagnosing LC was until recently problematic. Liver biopsy is not routine in Mongolia, only 40-60 are performed each year, mainly for diagnosis of HCC. This is due primarily to a shortage of interventional radiologists and liver pathologists. Noninvasive methods, such as Fibroscan, were not introduced until 2011, and > 500 patients have undergone this test to-date. Such methods will decrease the diagnostic errors associated with other nonspecific conventional methods such as ultrasonography (US) and increase the detection of LC, because both patients and doctors prefer noninvasive methods. Early diagnosis of HCC is of extreme importance in Mongolia.
There is a lack of established HCC surveillance systems in Mongolia. The number of high-quality US machines is very low, particularly in rural areas, and the quality of the US and examiners is unsatisfactory. Most family doctors do not classify their patients as high-, moderate-, and low-risk, which makes it difficult to refer patients for dynamic computed tomography (CT) due to a fear of frequent radiation exposure. Low-quality US machines operated by poorly trained technicians diagnose HCC lesions that have grown > 3 cm in most cases. Moreover, patient education is unsatisfactory. Many patients tend to seek medical attention only after the development of jaundice or liver failure.
High-resolution imaging diagnostic modalities, including CT and magnetic resonance imaging, are essential for a proper HCC diagnosis. Unfortunately, only two hospitals perform 2-3 phase contrast CTs at this time. In our recent as-yet unpublished study, the most-used diagnostic imaging modality for the first examination was US in all patients. The second-most common imaging modality was contrast-enhanced CT in 58 (44.9%) patients followed by contrast-enhanced CT and hepatic angiography in 32 (24.8%). The majority of patients had advanced HCC, with 63 (48.8%) in stage III and 49 (38.0%) in stage IV. It is important to note that no patient had stage I HCC and only 17 (13.2%) patients had stage II HCC.
The treatment modalities available in Mongolia are limited. In our study, surgical resection was performed in 16% of patients, and trans-arterial chemoembolization (TACE) was carried out in 27% of patients with multiple or large tumors. Radiofrequency ablation (RFA) is considered curative in conjunction with resection, but only 2% of patients received RFA as a first-line treatment and 55% received palliative care. Liver resection and TACE remain the mainstream treatment options in Mongolia (Fig. 7).
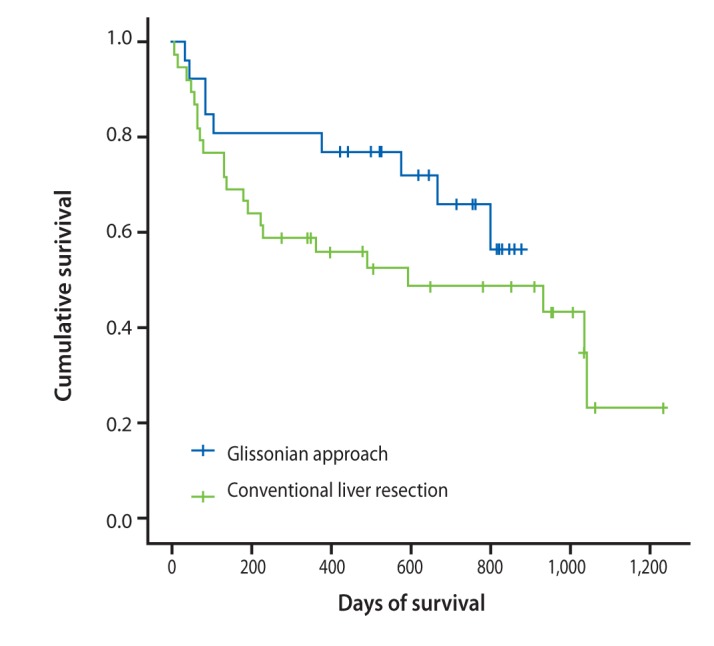
Two surgical methods are used for major hepatectomies: the conventional and Glissonian pedicle approaches (Fig. 8). The Glissonian pedicle approach was introduced in the last few years and has already produced positive results. While average blood loss was 358.5 mL in the Glissonian pedicle approach group, it was 747.5 mL during conventional liver resection. Patients who underwent liver resection using a Glissonian pedicle approach survived longer than those who received the conventional method (Fig. 9).
Viral hepatitis B, C, and alcohol consumption remain the main causes of chronic liver diseases, such as chronic hepatitis, LC, and HCC, in Mongolia. Insufficient data limits our knowledge of the exact burden of HDV as a cause of LC and liver tumors. Further study is needed to assess HDV seroprevalence in patients with LC and HCC. There has been no study of the genetic traits of Mongolians as a causative factor for HCC. Although the need for such research is debatable, genetic factors cannot be disregarded without careful investigation. The high incidence of HCC and end-stage liver disease must be addressed at all levels - etiology, diagnosis, treatment, and prevention. Delayed diagnosis contributes greatly to the high HCC and LC mortality rate in Mongolia. The national program, which includes high-risk patients and frequent follow-up examinations, must be implemented by the government as soon as possible. HCC and LC surveillance programs must be started countrywide immediately. LC previously raised little attention in Mongolia, but it certainly deserves more attention, because LC mortality is higher than that of gastric cancer. There is a need to train more liver pathologists and interventional radiologists, because there is currently no distinction made between early and advanced HCC in Mongolia. Liver fibrosis staging is not assessed by pathologists. With the introduction of noninvasive methods such as Fibroscan in 2012, LC and fibrosis diagnoses substantially improved. Advanced immunohistochemical and other staining methods have not been implemented. Only 40-60 liver biopsies are performed annually due to the shortage of interventional radiologists. Those biopsies are performed mostly for differentiation purposes of space occupying lesions.
Treatment options should be expanded. Approximately 60 RFA procedures are performed annually. The number of RFAs must be increased or the procedures should be performed in conjunction with other treatment methods such as TACE. Technical obstacles must be removed and the infrastructure available for RFA treatment should be improved.
Surgical resection must be provided as the first-line treatment option for patients who satisfy the criteria. The Glissonian pedicle approach shows better results in terms of mortality after treatment. Chemotherapy is one of the least effective treatment options for HCC. Sorafenib, though not officially registered in Mongolia, has been under clinical trial in five patients with unresectable HCC at NCC, and the results will be disclosed in the near future. Concerns of severe side effects and high cost as well as low survival benefit remain obstacles to HCC chemotherapy.
Palliative treatment is the only care for patients not qualified for surgery, RFA, or TACE, and 55% of HCC patients receive palliative care due to delayed diagnosis. Such patients often seek help from spiritual healers and take traditional herbal remedies. Some patients who qualify for surgical treatment prefer alternative medicine.
Liver transplantation is an attractive option for curative treatment but is currently not performed in HCC patients. Only five living donor liver transplantations (LDLT) have been performed, all for LC. The infrastructure, legal, and socioeconomic environment for LDLT must be improved greatly, so that as many as 500 additional procedures can be performed annually.
HCC can be greatly reduced with proper treatment of alcohol addiction and hepatitis viruses B, C, and D. Standard treatment for HCV, including peg-interferon plus ribavirin, was registered in Mongolia only 2-3 years ago. However, the price is exceedingly high for those with middle and lower class incomes. We calculated the total cost of HCV diagnosis and treatment on per patient and per country bases. An estimated 516,994 people are infected with HCV and 150 US$/person is needed for a full set of diagnostic tests. A total of 12,267 US$/person is needed for 48-week peginterferon treatment including 1 month of hospital stay, whereas classic interferon treatment costs 2,953 US$/person. Currently, with no insurance coverage for viral treatment, a total of 6.34 billion US$ is required to treat every citizen with pegylated interferon, and 1.52 billion US$ is needed to treat using conventional interferon therapy. The financial burden of HCV is so great that 41 life-years are needed for middle-class Mongolian patients to save enough money to have the latest hepatitis treatment. Poverty and insurance policies are the most to blame for this dire situation. HCV treatment will cost more than the country's gross domestic product, which stands at 4.2 billion US$ as of 2009. As more oral treatment agents become available in the coming years, it is likely that HCV treatment costs will increase before they decrease. Thus, it is crucial for the World Health Organization, pharmaceutical companies, and other international organizations to help countries like Mongolia fight HCV.
Treatment for HBV has improved recently. Lamivudine was registered 3 years ago, and tenofovir disoproxil fumarate is undergoing registration by the Mongolian Federal Drug Administration. Adefovir, telbivudine, and entecavir are not yet registered. Despite the affordable price of lamivudine for most patients, other regimens remain too expensive for low-income patients. Although there is no cure for HDV, upcoming trials show promise for peg-interferon only, peg-interferon in combination with tenofovir, or tenofovir alone treatments.
Alcohol consumption remains one of the major causes of HCC in Mongolia, and this issue has to be addressed by both doctors and the government. Poverty is the major cause of excess alcohol consumption, according to many studies. The flourishing alcohol business has to be controlled by the government and anti-alcohol laws must be implemented.
With the elimination of causes and improvements in diagnosis and treatment, we believe that a reduction in mortality from HCC and LC by at least by 30% is possible in the near future.
References
1. Ministry of Health. National Health Indicator 2009. 2009;Ulaanbaatar: Ministry of Health.
2. Nymadawa P. Hepatitis B vaccination: worldwide and in Mongolia 2004. In : 10th National Conference on "Current Topics of Virology"; Ulaanbaatar, Mongolia.
3. Baatarkhuu O, Kim DY, Ahn SH, et al. Prevalence and genotype distribution of hepatitis C virus among apparently healthy individuals in Mongolia: a population-based nationwide study. Liver Int 2008;28:1389–1395PMID : 18647237.


5. Stark K, Poggensee G, Hohne M, Bienzle U, Kiwelu I, Schreier E. Seroepidemiology of TT virus, GBC-C/HGV, and hepatitis viruses B, C, and E among women in a rural area of Tanzania. J Med Virol 2000;62:524–530PMID : 11074483.


6. Frank C, Mohamed MK, Strickland GT, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet 2000;355:887–891PMID : 10752705.


7. Tsatsralt-Od B, Takahashi M, Nishizawa T, Inoue J, Ulaankhuu D, Okamoto H. High prevalence of hepatitis B, C and delta virus infections among blood donors in Mongolia. Arch Virol 2005;150:2513–2528PMID : 16012782.


8. Tsatsralt-Od B, Takahashi M, Nishizawa T, Endo K, Inoue J, Okamoto H. High prevalence of dual or triple infection of hepatitis B, C, and delta viruses among patients with chronic liver disease in Mongolia. J Med Virol 2005;77:491–499PMID : 16254981.


9. Dondog B, Lise M, Dondov O, Baldandorj B, Franceschi S. Hepatitis B and C virus infections in hepatocellular carcinoma and cirrhosis in Mongolia. Eur J Cancer Prev 2011;20:33–39PMID : 21166097.


10. Kurbanov F, Tanaka Y, Elkady A, Oyunsuren T, Mizokami M. Tracing hepatitis C and Delta viruses to estimate their contribution in HCC rates in Mongolia. J Viral Hepat 2007;14:667–674PMID : 17697020.


11. Oyunsuren T, Kurbanov F, Tanaka Y, et al. High frequency of hepatocellular carcinoma in Mongolia: association with mono-, or co-infection with hepatitis C, B, and delta viruses. J Med Virol 2006;78:1688–1695PMID : 17063518.


12. Oyunsuren T, Sanduijav R, Davaadorj D, Nansalmaa D. Hepatocellular carcinoma and its early detection by AFP testing in Mongolia. Asian Pac J Cancer Prev 2006;7:460–462PMID : 17059345.

13. Jazag A, Puntsagdulam N. Demography and mortality of patients undergone liver resection due to HCC at National Cancer Center in Mongolia In : APASL 2nd Hepatocellular carcinoma Conference; 2011 Dec 1-3; Jeju, Korea.
Figure 1
Incidence of liver cancer per 100,000 population as of 2009 (Ministry of Health, Mongolia). Blue area indicates areas of high prevalence, light blue moderate prevalence, and white-blue low prevalence.
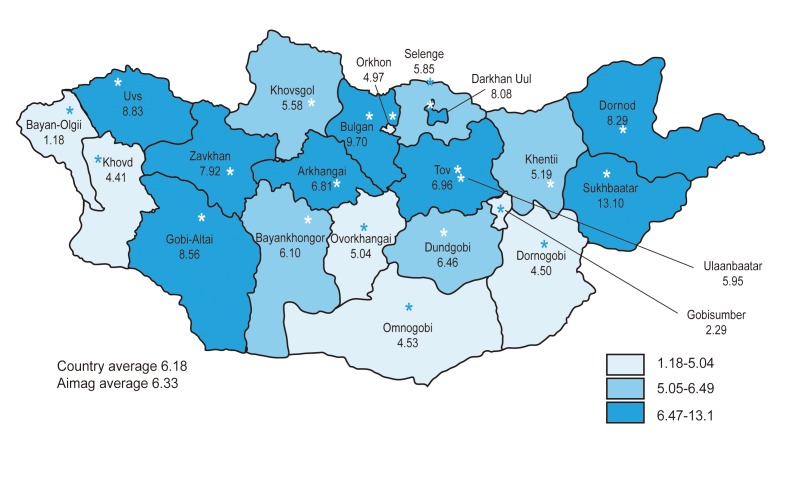
Figure 2
Liver cirrhosis-related deaths in Mongolia. Deaths due to liver cirrhosis during 2006-2010 are shown. Mortality from liver cirrhosis has been persistently high in the last 5 years.
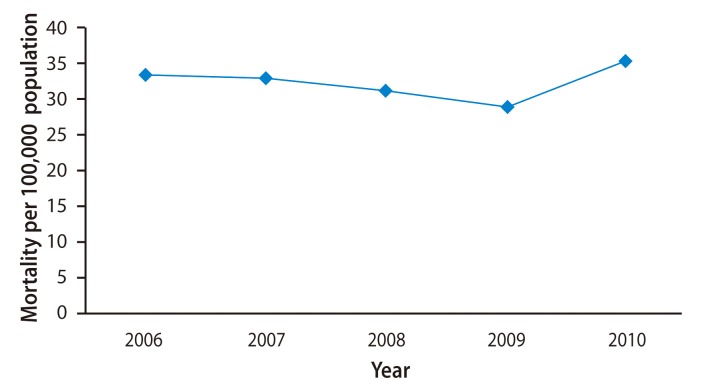
Figure 3
Viral causes of liver cirrhosis. Seroprevalence of hepatitis B virus surface antigen (HBsAg) and anti-HCV in patients with cirrhosis in Mongolia, 2000-2009. Anti-HCV, antibodies against hepatitis C virus; Anti-HCV+, seropositive for anti-HCV only; Dual+, seropositive for both HBsAg and anti-HCV; HBsAg+, seropositive for HBsAg only.
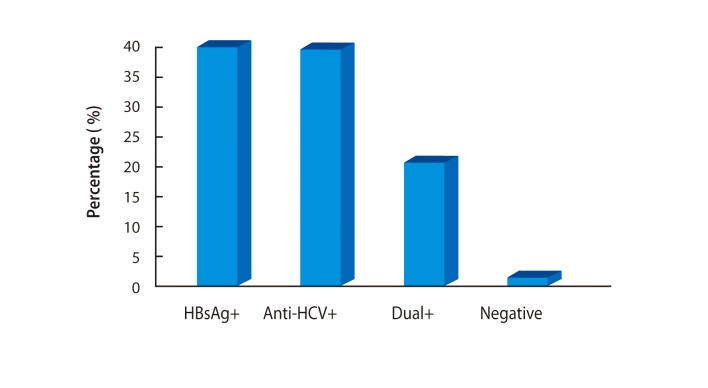
Figure 4
Increase in the incidence of hepatocellular carcinoma (HCC) in comparison with other cancers. Prevalence of HCC among different cancers (%) during three periods since 1975.
Figure 5
Incidence of hepatocellular carcinoma (HCC) since 1967. The increase in HCC cases over time in Mongolia (per 100,000 population) is shown.
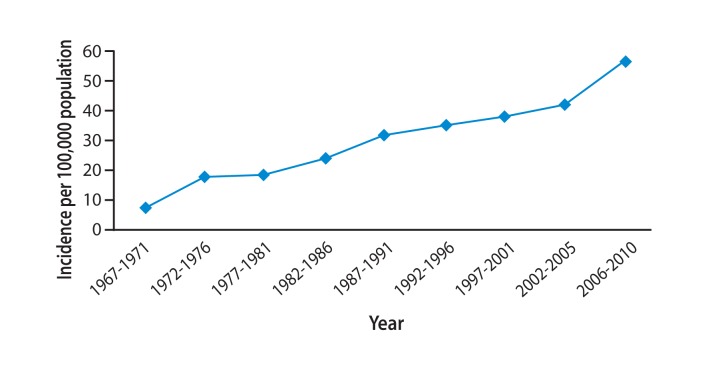
Figure 6
Age-adjusted incidence of hepatocellular carcinoma (HCC) in the Asia-Pacific region. Mongolia has the world's highest incidence of liver cancer, which is six times the global average and increasing.
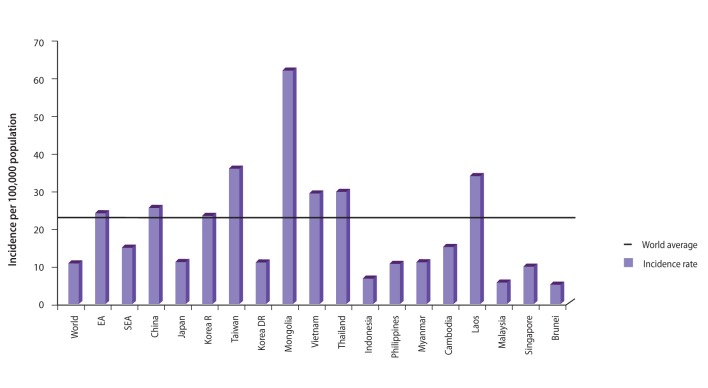
Figure 7
Management of hepatocellular carcinoma (HCC). According to National Cancer Center statistics, only 16% of patients with HCC are eligible for hepatoectomy, 29% are eligible for less-invasive procedures such as transarterial chemoembolization (TACE) or radio frequency ablation (RFA), whereas 55% are not eligible for any treatment.
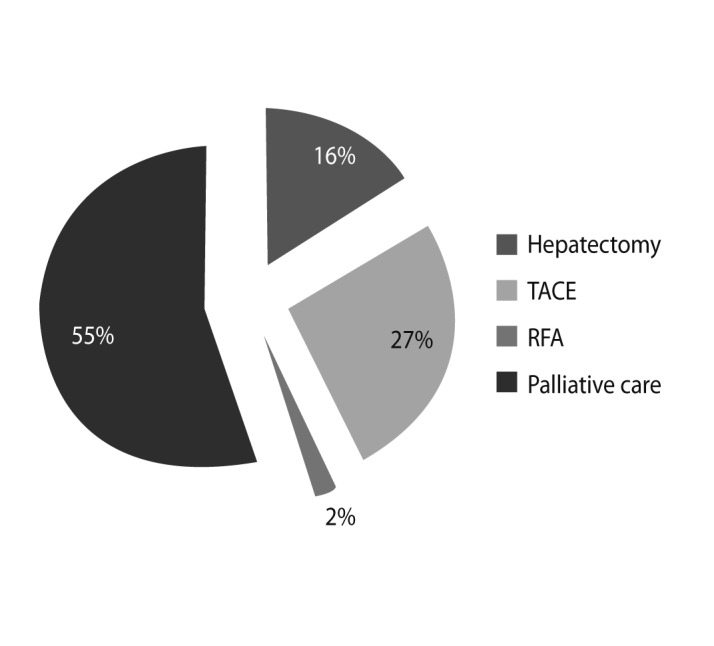
Figure 8
Shift in surgical method has resulted in few right hepatectomies. With implementation of the Glissonian technique in 2008 performance of major right liver resections is unnecessary in those patients whose cancers are located only in segments 5 and 8, and it is possible to preserve the anterior segment, which represents 33-35% of the liver, instead of removing 63-65%.
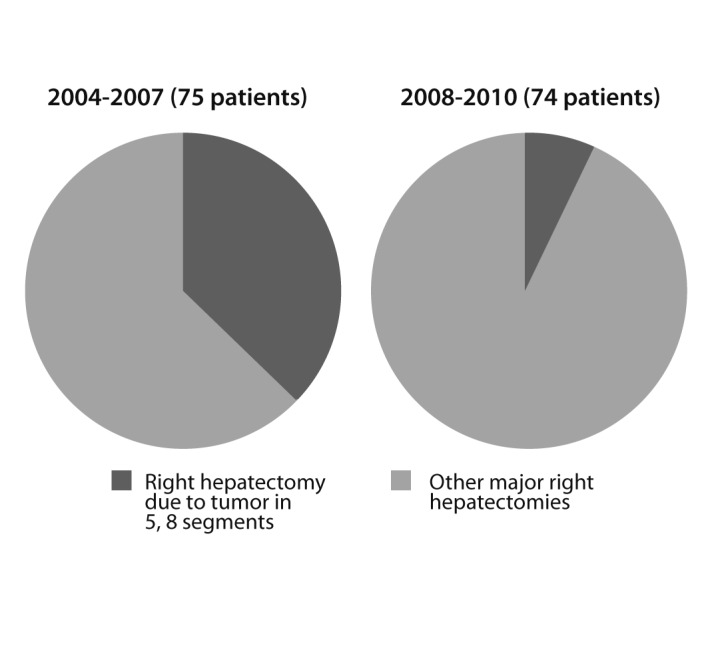
Figure 9
Survival rates of two surgical methods. The post-surgical survival rate (> 6 months) was 80.76% in patients who underwent the Glissonian approach, compared to 69.23% in patients who had conventional liver resection.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



