 |
 |
| Korean J Intern Med > Volume 26(4); 2011 > Article |
|
Abstract
The World Health Organization classifies lupus nephritis as class I to V or VI. However, a few cases of minimal change glomerulopathy have been reported in association with systemic lupus erythematosus (SLE). Mycophenolate mofetil has been shown to be effective for treatment of minimal change disease and lupus nephritis. A 24-year-old woman diagnosed with SLE five years prior to presentation complained of a mild generalized edema. The urinalysis showed microscopic hematuria and proteinuria. The assessed amount of total proteinuria was 1,618 mg/24 hours. A renal biopsy demonstrated diffuse fusion of the foot processes of podocytes on electron microscopy. Mycophenolate mofetil was started in addition to the maintenance medications of prednisolone 10 mg/day and hydroxychloroquine 400 mg/day. After six months of treatment, the microscopic hematuria and proteinuria resolved, and the total urine protein decreased to 100 mg/24 hours.
Mycophenolate mofetil (MMF) has been reported to be effective in the treatment of minimal change disease and lupus nephritis. However, there is no consensus on its use for minimal change disease especially in systemic lupus erythematosus (SLE).
A 24-year-old woman diagnosed with SLE five years previously and maintained on prednisolone 5 mg/day and hydroxychloroquine 400 mg/day complained of mild generalized edema.
At the time of the diagnosis of SLE, the patient was referred to the emergency room for cough, sputum production and bruising. Severe thrombocytopenia was associated with oral ulcerations, arthritis, high titer speckled fluorescent antinuclear antibody test (FANA), ds-DNA Ab, anti-Smith Ab, positive Venereal Disease Research Laboratory (VDRL) and normal bone marrow cellularity and maturity. These abnormalities normalized after treatment with intravenous immunoglobulin (IVIG) 400 mg/kg for five days, prednisolone 60 mg/day and hydroxychloroquine 400 mg/day.
In order to evaluate the newly developed generalized edema, the patient had a 24 hours urine test for total protein and a duplex scan for renal vascular patency and blood flow. The white blood cell (WBC) count was 4,800/µL, Hgb 13 g/dL, platelet 279,000/µL, aspartate aminotransferase/alanine aminotransferase (AST/ALT) 44/15 U/L, and blood urea nitrogen/creatinine (BUN/Cr) 9.8/0.6 mg/dL on blood testing. The urinalysis demonstrated 2 (++) proteinuria on albustick, and the total urine protein was 1,618 mg/24 hr. The duplex scan showed patent renal vasculature and normal flow.
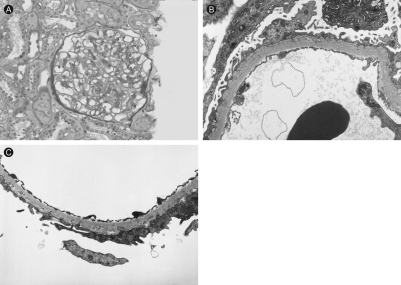
To evaluate the glomerular proteinuria in this patient with SLE a renal biopsy was performed. On light microscopy, no abnormalities were found; immunofluorescent staining showed normal findings (Fig. 1). However, electron microscopy revealed diffuse fusion and microvilli formation of the foot processes, no electron dense material in the mesangium, electrolucent and absorptive features of epimembranous deposits and tubulo-reticular bodies in the endothelial cell cytoplasm (Fig. 2). These findings were compatible with a podocytopathy. With no other possible causes for the glomerular podocytopathy the patient was diagnosed as minimal change disease associated with active SLE presenting with glomerular proteinuria and generalized edema.
To control minimal change disease, high-dose glucocorticoid therapy remains the mainstay. But because the patient had experienced glucocorticoid-related adverse effects such as severe edema, weight gain, gastro-intestinal disturbances and dermatopathy previously, prednisolone 10 mg/day and MMF, escalating up to 2.0 g/day, were added to the maintenance medications. The urine protein after three months of treatment decreased to 1 (+) on the albustick, and the 24 hours total urine protein was 945 mg. After six months, the WBC count was 7,350/µL, Hgb 12.9 g/dL, platelet 250,000/uL, AST/ALT 36/10 U/L, serum total protein 7.2 g/dL, serum albumin 3.8 g/dL, and BUN/Cr 10.5/0.7 mg/dL. The 24-hour total urine protein was 100 mg and the symptoms of generalized edema had resolved.
SLE is a prototype autoimmune disease involving multiple organs. Despite the potential to cause pathology in any organ, renal involvement in particular is known to be associated with active disease and is considered a major prognostic factor in patients with SLE.
Lupus nephritis is classified according to the World Health Organization consensus into class I to V or VI disease [1]. Glomerulonephritis is considered the result of immune injury mediated by lymphocytes. Anti-glomerular antibody formation, immune complex deposition on the basement membrane and anti-neutrophil cytoplasmic antibody activating neutrophils injure the glomerulus alone or in conjunction with autoantibodies and are important in the pathogenesis of this disease.
Minimal change disease is characterized by absence of abnormalities on light microscopy and absence of immunoglobulin deposits, in the glomerulus, on immunofluorescent staining. The differential diagnosis of these findings includes a malignancy such as Hodgkin's disease or drug toxicity such as non-steroidal anti-inflammatory drugs (NSAIDs) in adults. Only a few cases of minimal change disease have been reported to be associated with SLE [2,3].
In the setting of overproduction of autoantibodies and immune complexes, the pathogenesis of non-immune minimal change disease remains to be elucidated. Makino et al. [4] reported a case of minimal change nephritic syndrome associated with SLE and treated with methylprednisolone pulse therapy. Dube et al. [5] reported on seven patients with SLE and minimal change disease treated with prednisone and cessation of NSAIDs. Kraft et al. [6] reviewed glomerular podocytopathy in SLE patients with normal glomeruli by light microscopy, mesangial proliferative glomerulonephritis and focal segmental glomerulosclerosis (FSGS) on renal biopsy and concluded that the podocytopathy was the result of active lupus, rather than coexistence of two separate but concurrent diseases, SLE and minimal change glomerulopathy. Treatment of lupus nephritis requires aggressive therapy to reverse and prevent renal function from deteriorating to chronic kidney disease, especially in patients with class III and IV proliferative glomerulopathies where high dose glucocorticoids and immunosuppressants, such as cyclophosphamide and cyclosporine, are the standard treatments. Minimal change disease in adults is more likely to be resistant; patients are dependent on glucocorticoid treatment alone or combined with immunosuppressant therapy. However, even with adequate treatment, relapses are common.
Recently, MMF has been introduced as a promising therapeutic agent for the treatment of idiopathic nephritic syndrome even in cases of primary refractory nephritic syndrome [7]. Zhao et al. [8] reported that MMF was an effective and well tolerated immunosuppressive agent for patients with refractory nephritic syndrome in a prospective multicenter clinical observational study of 19 cases of refractory nephrotic minimal change disease and proliferative glomerulonephritis. Pesavento et al. [9] described the use of MMF in a case of frequently relapsing minimal change disease treated with cyclophosphamide therapy. Choi et al. [10] reported that empirical MMF therapy in the majority of patients with primary glomerulopathies was well tolerated. The treated patients achieved the goals of steroid withdrawal, improvement of nephrotic syndrome and stabilization of renal function in forty-six patients with biopsy proven primary glomerulopathies on MMF for more than three months. MMF can be a treatment alternative to the standard cyclophosphamide regimes in refractory cases. However, there are currently no consensus guidelines for MMF treatment of lupus nephritis and minimal change disease, especially where it is associated with SLE. We successfully treated a patient who presented with minimal change disease of non-nephrotic range proteinuria in SLE with MMF.
References
1. Churg J, Bernstein J, Glassock RJ. Renal Disease: Classification and Atlas of Glomerular Diseases. 1995;2nd ed. New York: Igaku-Shoin.
2. Wang YT, Chou HH, Chen FF, Chen MJ, Chiou YY. A case of minimal-change nephrotic syndrome in pediatric lupus erythematosus: just a coincidence? Lupus 2006;15:244–247PMID : 16686266.


3. Joo HR, An WS, Lee SW, et al. A case of minimal change nephrotic syndrome associated with systemic lupus erythematosus. Korean J Med 2003;65:251–255.
4. Makino H, Haramoto T, Shikata K, Ogura T, Ota Z. Minimal-change nephritic syndrome associated with systemic lupus erythematosus. Am J Nephrol 1995;15:439–441PMID : 7503146.


5. Dube GK, Markowitz GS, Radhakrishnan J, Appel GB, D'Agati VD. Minimal change disease in systemic lupus erythematosus. Clin Nephrol 2002;57:120–126PMID : 11863121.


6. Kraft SW, Schwartz MM, Korbet SM, Lewis EJ. Glomerular podocytopathy in patients with systemic lupus erythematosus. J Am Soc Nephrol 2005;16:175–179PMID : 15548564.


7. Baglio V, Pecci G, Gangemi C, Barresi G, Morabito S, Pierucci A. Failure of mycophenolate mofetil therapy in primary refractory nephrotic syndrome. J Nephrol 2006;19:819–824PMID : 17173257.

8. Zhao M, Chen X, Chen Y, et al. Clinical observations of mycophenolate mofetil therapy in refractory primary nephritic syndrome. Nephrology (Carlton) 2003;8:105–109PMID : 15012724.


Figure 1
Kidney biopsy. No specific pathology was found on light microscopy (Periodic acid-Schiff stain, ×200).
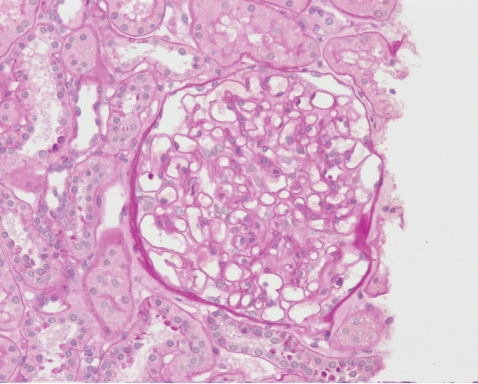
Figure 2
Kidney biopsy. The electron microscopy showed diffuse fusion of the foot processes, microvilli formation of foot processes, no electron dense material in the mesangium, electrolucent and absorptive features of epimembranous deposits and tubulo-reticular bodies in the endothelial cell cytoplasm (Uranyl acetate and lead citrate double stain; A: × 2,000; B, C: × 5,000).



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



