1. Lee SM, Yoo CG. Acute respiratory distress syndrome. Korean J Med 2005;68:476ŌĆō486.

2. Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818ŌĆō824PMID : 7509706.


3. Maunder RJ, Shuman WP, McHugh JW, Marglin SI, Butler J. Preservation of normal lung regions in the adult respiratory distress syndrome: analysis by computed tomography. JAMA 1986;255:2463ŌĆō2465PMID : 3701964.


4. Gattinoni L, Mascheroni D, Torresin A, et al. Morphological response to positive end expiratory pressure in acute respiratory failure: computerized tomography study. Intensive Care Med 1986;12:137ŌĆō142PMID : 3525633.


5. Piehl MA, Brown RS. Use of extreme position changes in acute respiratory failure. Crit Care Med 1976;4:13ŌĆō14PMID : 1253612.


6. Chatte G, Sab JM, Dubois JM, Sirodot M, Gaussorgues P, Robert D. Prone position in mechanically ventilated patients with severe acute respiratory failure. Am J Respir Crit Care Med 1997;155:473ŌĆō478PMID : 9032181.


7. Blanch L, Mancebo J, Perez M, et al. Short-term effects of prone position in critically ill patients with acute respiratory distress syndrome. Intensive Care Med 1997;23:1033ŌĆō1039PMID : 9407238.


8. Servillo G, Roupie E, De Robertis E, et al. Effects of ventilation in ventral decubitus position on respiratory mechanics in adult respiratory distress syndrome. Intensive Care Med 1997;23:1219ŌĆō1224PMID : 9470076.


9. Pelosi P, Tubiolo D, Mascheroni D, et al. Effects of the prone position on respiratory mechanics and gas exchange during acute lung injury. Am J Respir Crit Care Med 1998;157:387ŌĆō393PMID : 9476848.


10. Martinez M, Diaz E, Joseph D, et al. Improvement in oxygenation by prone position and nitric oxide in patients with acute respiratory distress syndrome. Intensive Care Med 1999;25:29ŌĆō36PMID : 10051075.


11. Nakos G, Tsangaris I, Kostanti E, et al. Effect of the prone position on patients with hydrostatic pulmonary edema compared with patients with acute respiratory distress syndrome and pulmonary fibrosis. Am J Respir Crit Care Med 2000;161:360ŌĆō368PMID : 10673172.


12. Rialp G, Betbese AJ, Perez-Marquez M, Mancebo J. Short-term effects of inhaled nitric oxide and prone position in pulmonary and extrapulmonary acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;164:243ŌĆō249PMID : 11463595.


13. Gattinoni L, Tognoni G, Pesenti A, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001;345:568ŌĆō573PMID : 11529210.


14. Mancebo J, Fernandez R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med 2006;173:1233ŌĆō1239PMID : 16556697.


15. Fernandez R, Trenchs X, Klamburg J, et al. Prone positioning in acute respiratory distress syndrome: a multicenter randomized clinical trial. Intensive Care Med 2008;34:1487ŌĆō1491PMID : 18427774.


16. Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest 1998;114:541ŌĆō548PMID : 9726743.


17. Kress JP, Hall JB. Sedation in the mechanically ventilated patient. Crit Care Med 2006;34:2541ŌĆō2546PMID : 16932231.


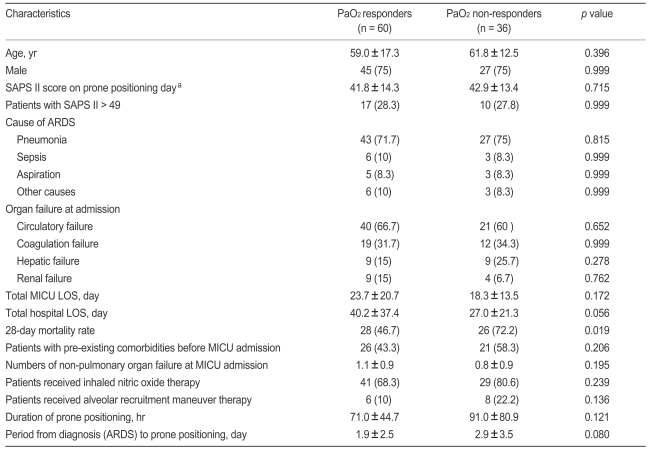
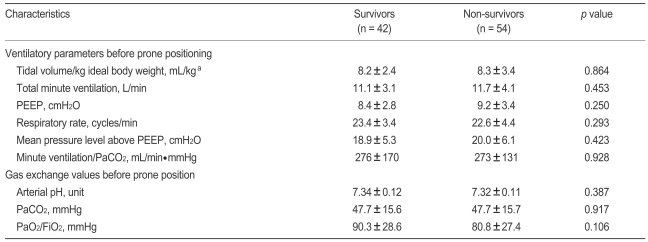
18. Gattinoni L, Vagginelli F, Carlesso E, et al. Decrease in PaCO2 with prone position is predictive of improved outcome in acute respiratory distress syndrome. Crit Care Med 2003;31:2727ŌĆō2733PMID : 14668608.


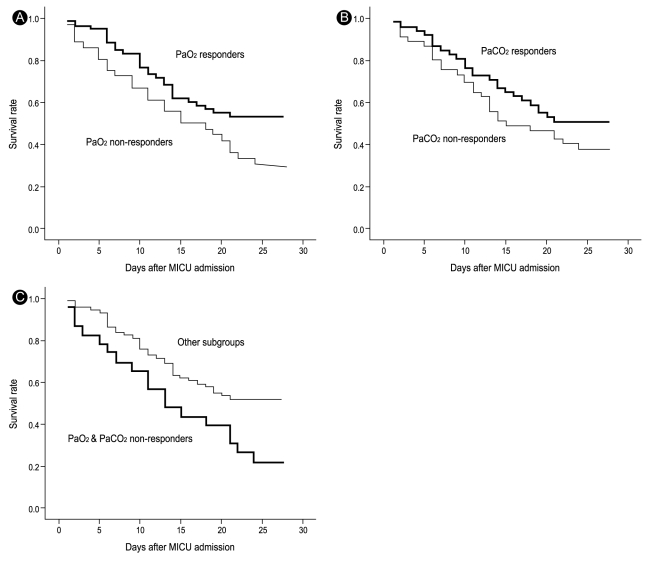
19. Lemasson S, Ayzac L, Girard R, Gaillard S, Pavaday K, Guerin C. Does gas exchange response to prone position predict mortality in hypoxemic acute respiratory failure? Intensive Care Med 2006;32:1987ŌĆō1993PMID : 17019539.


20. Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993;270:2957ŌĆō2963PMID : 8254858.


21. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome: the Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301ŌĆō1308PMID : 10793162.


22. Lim CM, Jung H, Koh Y, et al. Effect of alveolar recruitment maneuver in early acute respiratory distress syndrome according to antiderecruitment strategy, etiological category of diffuse lung injury, and body position of the patient. Crit Care Med 2003;31:411ŌĆō418PMID : 12576945.


23. Sevransky JE, Levy MM, Marini JJ. Mechanical ventilation in sepsis-induced acute lung injury/acute respiratory distress syndrome: an evidence-based review. Crit Care Med 2004;32(11 Suppl):S548ŌĆōS553PMID : 15542963.


24. Guerin C, Gaillard S, Lemasson S, et al. Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA 2004;292:2379ŌĆō2387PMID : 15547166.


25. Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med 2002;30:119ŌĆō141PMID : 11902253.









 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



