INTRODUCTION
The acidic saline animal model of pain is thought to mimic human chronic pain syndromes such as fibromyalgia (FM). In this model, repeated intramuscular (IM) injections of acidic saline produce widespread hyperalgesia that persists without evidence of significant peripheral tissue damage or inflammation, and is believed to be centrally maintained [1]. IM acid injections alter the basal pattern of brain activation in rats [2]. The overwhelming reduction of regional cerebral blood flow (rCBF) in multiple brain structures following acid injections is consistent with reports of decreased rCBF in unstimulated patients with FM [3,4].
Milnacipran is a dual norepinephrine and serotonin reuptake inhibitor approved for the treatment of depression in Europe and Asia, but it is currently unavailable in the US [5]. The results of a 12-week, randomized, placebo-controlled, dose-finding trial for milnacipran have been reported; 25-200 mg/day of milnacipran or placebo was given as a daily or twice-daily regimen during the first 4 weeks followed by 8 weeks at a stable dose [6]. Most FM patients titrated to the highest dose of 200 mg/day whether they were dosed once or twice daily. A significantly higher percentage of patients receiving milnacipran twice daily (37%) than placebo (14%) reported a reduction in weekly average pain scores.
Data from imaging studies are beginning to explain clinical observations that opioid analgesics may be less effective or require higher dosing in patients with FM because of the reduced availability of opioid binding sites [7]. Many studies have shown that compounds known to block monoamine uptake, such as tricyclic antidepressants, potentiate the antinociceptive effects of opioids [8,9]. Tramadol proved useful in patients with FM in a randomized, placebo-controlled study [10].
Data regarding the efficacy of pharmacologic combination treatment for FM are very limited. To our knowledge, no study has examined pharmacologic combination treatment in an acidic saline pain model. To provide a basis for pharmacologic combination treatment for human FM, we studied the efficacy of milnacipran in conjunction with tramadol for hyperalgesia in an acidic saline animal model of pain.
METHODS
All experiments were approved by the Animal Care and Use Committee at Dongguk University and were in accordance with the guidelines of the International Association for the Study of Pain.
Twenty male Sprague-Dawley rats weighing 250-320 g were used in these experiments. The rats were maintained on a 12-h dark/light cycle with ad libitum access to standard rat chow and water, and then brought to the behavioral testing room an hour before testing for acclimatization. Behavioral tests were usually performed between 10:00 and 15:00. The left gastrocnemius muscle was injected with 100 ┬ĄL of saline at pH 4.0 under brief isoflurane (2%) anesthesia on days 0 and 5.
Rats with acidic saline injection were separated into four study subgroups according to intraperitoneal injection regimen (group 1: milnacipran 60 mg/kg alone, group 2: milnacipran 40 mg/kg + tramadol 20 mg/kg, group 3: milnacipran 40 mg/kg + tramadol 40 mg/kg, group 4: saline). After measurement of the control pre-drug pain thresholds, rats were injected intraperitoneally with either of the following regimens; saline, milnacipran 60 mg/kg alone, and milnacipran 40 mg/kg in combination with tramadol 20 or 40 mg/kg. When milnacipran and tramadol were administered in combination, each agent was injected to the different regions of abdomen to prevent any possible incompatibility. Paw withdrawal thresholds to paw pressure were again measured 30 min, 120 min, and 5 days after injection. Milnacipran was a generous gift of Bukwang Pharma (Republic of Korea). Tramadol was also a generous gift of Janssen Korea Ltd. (Republic of Korea).
Ipsilateral and contralateral paw withdrawal responses were measured in response to mechanical stimuli on days 0 (baseline), 7, and 12. The tests were performed by an experimenter blinded to the treatment groups. Nociceptive thresholds, expressed in grams (g), were measured using a Dynamic Plantar Aesthesiometer (Ugo Basile, Italy) by applying increasing pressure to the right or left hind paw until the rat withdrew the paw. A maximal cut-off value of 50 g was used to prevent tissue damage.
The threshold was tested five times for each time point and the mean values were used in the analysis. One-way analysis of variance (ANOVA) followed by post hoc analysis for multiple comparisons were used to evaluate the differences between groups for each time point.
RESULTS
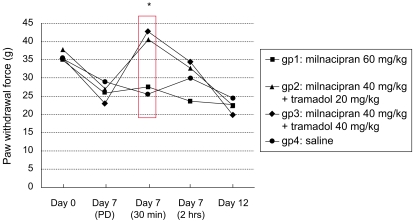
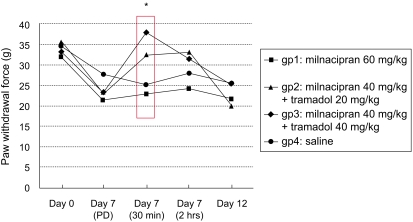
Compared to baseline (day 0), acid injections produced mechanical hyper-responsiveness on day 7 (pre-drug administration, p<0.05, Fig. 1 and 2). However, when milnacipran and tramadol were administered in combination via intraperitoneal injection, a potent antihyperalgesic effect was observed in the injected paw (p=0.001, Fig. 1). A similar antihyperalgesic effect was also observed in the contralateral paw when the two drugs were used in combination (p=0.012, Fig. 2). However, these antihyperalgesic effects were observed only at 30 min after the combination treatments.
An additional increase in the pain threshold of the contralateral paw was observed at 30 min after treatment when 40 mg/kg of tramadol was combined with milnacipran 40 mg/kg (Fig. 2). However, this increase was not statistically significant.
DISCUSSION
In the present study, unilateral IM injections of acidic saline produced bilateral mechanical hyperresponsiveness in Sprague-Dawley rats. In this model, a potent antihyperalgesic effect was observed when milnacipran (40 mg/kg) was combined with tramadol at a dose of 20 or 40 mg/kg. An increase in the pain threshold was observed not only in the paws on the injected side, but also in paws on the non-injected side of rats with hyperalgesia.
The affinity of antidepressants for opioid receptors is low [11]; therefore agonist-like effects of antidepressants on opioid receptors are unlikely [12]. Apart from a direct mechanism by which opioid receptors may be engaged by milnacipran, an indirect pathway may be involved in the observed increase in pain threshold in this study, perhaps via inhibition of the release of endogenous opioid peptides [13]. Several animal studies showed an increase in central nervous system (CNS) opioid peptide levels or immunoreactivity after antidepressant treatment [14,15]. However, the mechanism by which antidepressants may increase the release of opioid peptides is yet to be identified. The results of previous studies, as well as those of the present study, indicate that further investigation is necessary to obtain more detailed information regarding the interaction of milnacipran and other non-tricyclic antidepressants with opioidergic receptors.
Although there is no established experimental animal model for FM, the acidic saline animal model of pain has been suggested to mimic FM [1]. Hyperalgesia is thought to result from changes occurring in multiple regions of the CNS, a massive integrated neural network [16,17]. Thus, the pain modulation system of the CNS should be investigated more widely. Although electrophysiological techniques are useful to investigate the pain modulation system, the identification of molecules that function in the system remains difficult. Kim et al. [18] reported that, compared to control animals, acid-injected rats showed increased expression of several pain-associated molecules in specific structures of the CNS. In addition, unilateral IM injections of acidic saline altered the basal pattern of activation in multiple CNS structures [2]. These data support the validity of the acidic saline assay as a model of FM, and provide a foundation for future analyses of the contribution of specific brain structures and cellular mechanisms to the development and maintenance of central pain syndromes.
Pregabalin has been shown to be efficacious in other pain conditions, including postoperative pain and FM. Specifically, in people with FM, pregabalin (450 mg/day) reduced pain, improved sleep, and increased quality of life [19]. More recently, it was reported that pregabalin reduced muscle and cutaneous hyperalgesia in an acidic saline model of rats [20]. Data regarding the effect of pharmacologic combination treatment in patients with FM are limited, and no study regarding pharmacologic combination treatment in an acidic saline model has been published to date. In this study, we demonstrate that a combined treatment regimen consisting of milnacipran and reduced-dosage tramadol provides adequate additive or supra-additive therapeutic effects with minimal adverse effects. Efforts to define optimal drug combinations for the relief of FM are continuing, and studies of other drugs in combination with antidepressants are potentially valuable. Therefore, further research is required to determine the efficacy of these combinations in patients with FM.
In conclusion, we demonstrated that the antihyperalgesic effect of milnacipran was potentiated when administered in combination with tramadol in an animal model of FM. Further research is required to determine the efficacy of various combination treatments in FM in humans.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





