 |
 |
| Korean J Intern Med > Volume 22(2); 2007 > Article |
|
Abstract
Background
Pulmonary damage resulting from lipid peroxidation is a principal effect of paraquat intoxication. The host-defense functions of surfactant are known to be mediated by the surfactant proteins A and D (SP-A and SP-D, respectively). The primary objective of this study was to evaluate the variations over time in levels of surfactant protein and lipid peroxidation (LPO) in lung tissue following free-radical-induced injury.
Methods
42 adult, male, Sprague-Dawley rats were administered intraperitoneal injections of paraquat (35 mg/kg body weight). SP-A and SP-D levels were determined via Western blot. LPO in the left lung homogenate was measured via analyses of the levels of thiobarbituric acid-reactive substances.
Results
LPO levels peaked at 6 hours, with no associated histological changes. SP-D levels increased until hour 12 and declined until hour 48; SP-D levels subsequently began to increase again, peaking at hour 72. SP-A levels peaked at hour 6, declining thereafter.
Conclusions
We suggest that in the early phase of paraquat injury, SP-D levels reflect alveolar damage and that de novo synthesis of SP-D takes 72 hours. Levels of SP-A, on the other hand, reflect abnormalities in the surfactant system in the late stage of paraquat intoxication. Surfactant proteins may play a role in protecting the lungs from reactive oxygen injury. A time-dependent variation has been observed in the levels of surfactant proteins A and D following paraquat injury, and it has been suggested that these proteins play a role in the protection of lung tissue against ROS-induced injuries.
Paraquat dichloride (1,1'-dimethyl-4,4'-bipyridilium dichloride; methylviologen) is an effective and widely-employed herbicide. Paraquat is toxic in humans, with the lungs being the principal target organ, probably as the consequence of the existence of an active uptake system1, 2). The toxic effects of paraquat on the lungs result in pulmonary edema, hypoxia, respiratory failure, and pulmonary fibrosis. Mortalities as the result of paraquat poisoning are generally attributed to extensive pulmonary injury3). Respiratory distress occurring during the early stages of paraquat poisoning may be attributable to impairments of the surfactant system, probably resulting not only from the paraquat itself, but also from the effects of oxygen free radicals. Pulmonary fibrosis in the late stage of paraquat poisoning is generally observed 531 days after poisoning. Manktelow demonstrated that paraquat induces changes in the lung that are comparable to the lesions observed in cases of respiratory distress syndrome4). Pulmonary surfactant is composed of 90% lipid and 10% protein. Four surfactant-associated proteins exist: surfactant protein A (SP-A), surfactant protein B (SP-B), surfactant protein C (SP-C), and surfactant protein D (SP-D). Evidence has accumulated over the past 1015 years that SP-A and SP-D function as part of the first line of immune defense in the lung, by binding to pathogens, thereby promoting their phagocytosis and killing by phagocytes5). SP-A-knockout mice evidence normal lung histology, but lack SP-A, as has been predicted, exhibiting an increased susceptibility to pulmonary infection by bacteria and viruses6). By way of contrast, murine SP-D deficiency unexpectedly induces spontaneous emphysematous change and the development of pulmonary fibrosis, thereby revealing the crucial role for SP-D in the control of lung inflammation7, 8). The pathology and biochemical characterization of paraquat-induced lung-injury models have been previously reported9, 10). The present study was undertaken in order to characterize the variations over time in surfactant protein levels, and to determine the levels of lipid peroxidation in lung tissue following free-radical-induced injury.
Adult male Sprague-Dawley rats (approximate body weight 200-220 g), which were maintained on a standard laboratory diet and water, were employed in this study. The rats received intraperitoneal (ip) injections with paraquat (35 mg/kg body weight, Sigma Chemical, St. Louis, MO, USA) in 1 ml saline. The animals were anesthetized via ip injections of phenobarbital (50 mg/kg body weight) at time 0 (control, immediately after paraquat injection), 6 hours, 12 hours, 24 hours, 2 days, 3 days, or 5 days after the paraquat injection. They were then exsanguinated via the abdominal aorta, and the chest wall was opened.
Three aliquots of 7.5 mL saline at 4℃ were used to fill the lungs. Each aliquot was flushed into and out of the airways three times. The recovered volumes (about 85% of the injected volume) from all animals from the same experimental group (control, and 6 hours, 12 hours, 24 hours, 2 days, 3 days, or 5 days postinjection) were pooled.
Protein concentrations in the lavage fluid were measured via the Bradford method11) using bovine serum albumin as a standard.
BAL fluid was collected from paraquat-treated rats and diluted to 15 mL in BNC buffer (10 mM sodium borate, pH 7.4, 150 mM NaCl, 3 mM CaCl2). The sample was centrifuged for 10 minutes at 250 g at 4℃, followed by 2 hours of centrifugation at 27,000 g, and the resultant pellet was resuspended in 100 l of BNC buffer and stored at 20℃. Sodium dodecyl sulfate- polyacrylamide gel electrophoresis and Western blot analysis were then conducted. Samples containing 1 g of BAL were boiled for 4 minutes and applied to 12% NuPAGE Novex Bis-4,7-diphenyl-1,10-phenanthroline (Tris) gels using the XCell SureLock Mini-Cell system (Invitrogen Life Technologies), in accordance with the manufacturer's instructions. After electrophoresis, gels were transferred onto polyvinylidine difluoride membranes at 35 V for 2 hours, followed by 1 hour of blocking in phosphate-buffered saline (PBS) with 0.5% bovine serum albumin and 0.02% Tween 20. The membranes were then incubated overnight at 4℃ in a primary antibody against either SP-A or SP-D (Santa Cruz) diluted to 1:500 with PBS. The membranes were then incubated for 1 hour in peroxidase-conjugated secondary antibody diluted to 1:1,000 with PBS at room temperature. The blots were developed with an enhanced chemiluminescence detection system (ECL Plus from Amersham Bioscience)10).
Left lung homogenate and determination of tissue thiobarbituric acid-reactive substances (TBA-R S) contents
After the lungs were perfused free of blood and harvested for lavage fluid, the left lung was placed in a tube and rapidly frozen in enzyme-linked immunosorbent assay buffer and then stored at 70℃. For analysis, the tissue was placed in homogenizing buffer (50 Mm Tris HCl, pH 7.5, containing 1 Methylenediaminetetraacetic acid, 2 mM phenylmethylsulfonyl fluoride, and 2.5 mM N-ethylmaleimide) at a defined ratio of 1 g of lung tissue to 9 mL of homogenizing buffer. The lung tissue was then homogenized on ice with a Polytron (Brinkman Instruments, Westbury, NY, USA). The lung homogenates were spun for 5 minutes at 300g to sediment the tissue debris. The fluorometric method of Okhawa, (excitation at 532 nm; emission at 551 nm) was utilized in order to determine the tissue TBA-RS contents11). A standard curve was prepared with the aid of tetramethoxypropane (Sigma), which under the assay conditions is hydrolyzed to malondialdehyde (MDA). The detection limit was 0.8 mmol MDA-thiobarbituric acid/ml. TBA-RS levels are expressed as nmol/g of tissue. A light microscopy procedure was then performed. The rats were anesthetized with sodium phenobarbital, after which a thoracostomy and BAL were conducted, and the right lung was sectioned. The right lung was fixed with an intratracheal instillation of 4% formaldehyde and 1% glutaraldehyde in 0.1 M phosphate buffer at a pH of 7.2 at room temperature. For light microscopy, the pulmonary tissue blocks were embedded in paraffin and sectioned at 4 m. The sections were mounted on glass slides and stained with hematoxylin and eosin.
Data are expressed as meansSD. A probability value of p<0.05 was considered to be indicative of statistical significance, with all statistical analyses conducted using SPSS for Windows (version 12.0, Chicago, Illinois, USA). In cases in which an F test indicated homogenous variances, the Student's t-test was applied; if not, a modified Student's t-test was utilized. Differences between groups were tested via ANOVA. For multiple comparisons, Tukey's test was applied after ANOVA.
Eight of the 42 paraquat-treated rats died before sacrifice. The timing and number of deaths were as follows: <24 hours, 0; 2448 hours, 2; 4872 hours 2; >72 hours, 4). Some of the paraquat-treated rats evidenced hypokinesia and anorexia.
As is shown in Figure 1, SP-D levels in the lung lavage increased until hour 12 after the paraquat injection. At 24 hours, SP-D levels again declined to near control levels, but subsequently increased again, achieving peak levels at 72 hours. Differences between groups were not statistically significant (p=0.66). SP-D levels were higher at 72 hours' posttreatment than in the control animals, or at 24 hours and 48 hours posttreatment (p<0.05).
As is shown in Figure 2, SP-A levels in lung lavage fluid achieved peak levels at 6 hours after paraquat injection, after which they declined to levels below normal (control). Differences between groups were statistically significant (p<0.05) as follows: control, 6-hour, and 12-hour posttreatment groups were significantly different from the 24-hour, 48-hour, and 72-hour posttreatment groups (Tukey's test).
Tissue TBA-RS contents differed significantly between the groups, achieving peak levels at 6 hours' postinjection. TBA-RS levels in the control group differed significantly from those of the 6-hour postinjection group (p<0.05) (Figure 3).
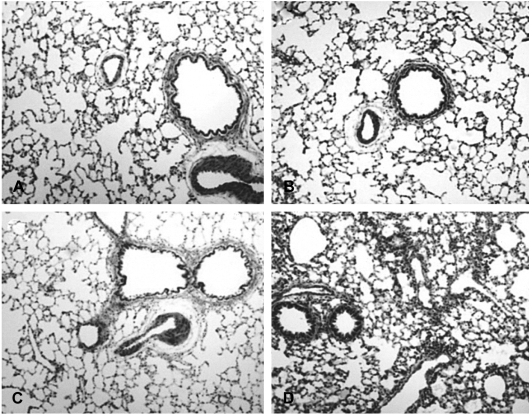
As is shown in Figure 4, the lung tissue in paraquat-treated rats evidenced inflammatory cell infiltration around the bronchioles at 12 hours' postinjection. Inflammatory cell infiltration and hemorrhaging were noted 72 hours after paraquat injury.
Paraquat is a widely used and effective herbicide, which evidences a broad spectrum of activity. Experimental studies have indicated that it accumulates in the epithelial cells of the lung and kidney, eventually resulting in pulmonary fibrosis and acute renal failure. Lung damage after paraquat poisoning appears to be secondary to derangement of the pulmonary surfactant system4, 12-14), probably resulting not only from selective paraquat-induced injury to type 1 and type 2 cells, but also from the increased permeability of alveolar capillaries after oxygen-radical-induced injury to endothelial cells15). The characteristic proliferative lesions associated with paraquat poisoning, namely pulmonary fibrosis, may not become evident until 1 week after injury, whereas damage resulting from free radicals commences immediately16). The destructive phase is characterized by the loss of type I and type II alveolar cells, loss of surfactant, infiltration by inflammatory cells, and hemorrhaging. The subsequent proliferative phase is characterized by a loss of alveolar space. The fibrosis, which is mediated in part by cytokines, is not specific to paraquat-induced injury, but rather is observed in response to the acute alveolitis induced by a variety of pulmonary toxins17).
The overwhelming of tissue defense mechanisms by excessive free-radical production may induce a lipid peroxidation process, thereby resulting in extensive membrane damage. Several markers have been proposed for the detection of lipid peroxidation, but measurements of TBA-RS are probably the most widely used technique. In the present study, measurements of tissue TBA-RS levels showed that oxidative injury began at an early stage, at which point it induced no histological changes. It has been established that the surfactant system is one of the targets of paraquat toxicity. In a variety of animal studies, inhalation of surfactant proved effective in preserving lung compliance after paraquat poisoning18). Surfactant is composed principally of phospholipids, which are essential for reducing surface tension at the airliquid interface of the lung. Approximately 10% of surfactant is protein, and four surfactant proteins have been identified: SP-A, SP-B, SP-C, and SP-D. SP-B and SP-C are small and extremely hydrophobic. SP-B is crucial for the ability of surfactant to reduce surface tension19). The host-defense functions of surfactant are mediated principally by SP-A and SP-D, both of which are members of the collectin protein family20).
In the study presented herein, the concentration of SP-D in the lung was elevated until 12 hours after the paraquat injection. After 1 day, the level of SP-D had declined to below control levels; they then began to increase again, achieving peak levels at 72 hours' postinjection. We suggest that in the early phase after paraquat injury, the level of SP-D is reflective of the alveolar damage induced by excessive free-radical production. During this phase, the SP-D levels measured may originate from endogenous stores; de novo synthesis of SP-D takes 72 hours. By way of contrast, SP-A levels peaked at 6 hours' postinjection, subsequently declining to below control levels. This indicates that SP-A, which is intimately associated with surfactant lipid membranes and aggregates21), performs a role different from that suggested by the lung model of paraquat injury. Experimental data imply that SP-A preserves the surface activity of surfactant, protects it against the effects of serum protein inhibitors22), contributes to the stability of surfactant aggregates including tubular myelin23), and inhibits phospholipase A2 activity24, 25). SP-D, however, appears to be a prerequisite for the maintenance of surfactant homeostasis and lung structure8). In the study presented herein, the effects of paraquat on SP-A levels began only after 6 hours' post-injection, thereby suggesting that this surfactant protein is affected in the late stage after paraquat intoxication.
Several factors may be responsible for the variable effects of paraquat intoxication on SP-A and SP-D over time. First, SP-A and SP-D function as endogenous antioxidants. Paraquat elicits the generation of reactive oxygen species (ROS), which destroy type II pneumocytes. The elevation of surfactant protein levels may protect the lung tissue against paraquat-induced ROS. Bridges previously demonstrated that pulmonary SP-A and SP-D are potent endogenous inhibitors of lipid peroxidation and oxidative cellular injury26). SPD attenuates alveolar macrophage apoptosis in vivo27), evidences antioxidant properties, and is crucial to the modulation of apoptotic cell numbers in the lung, thereby suggesting a protective function for this surfactant protein in the prevention of abnormal alveolar remodeling after oxidative lung injury, and may also play a role in immune and lung developmental processes.
Second, in the early stages of paraquat intoxication, SP-A and SP-D levels reflect the extent of lung injury. Pan previously demonstrated that serum SP-D is a marker for lung injury in rat28). In cases in which paraquat, bleomycin, HCl, or keratinocyte growth factor are instilled into the lungs, serum and pulmonary levels of SP-D are elevated in BAL fluid. SP-D levels may reflect the extent of lung injury in the early stages of paraquat poisoning. In the later stages, levels of SP-A may be the result of destructive lung injury and fibrosis. Exogenous surfactant therapy has proven effective in several animal models of acute lung injury29, 30), and has also been evaluated in humans suffering from sepsis-induced acute respiratory distress syndrome (ARDS).
However, clinical responses to exogenous surfactant therapy have varied substantially. A large multicenter clinical trial using aerosolized artificial surfactant was not determined to benefit patients with sepsis-induced ARDS29). The lack of response to artificial surfactant may have been resultant from the absence of surfactant apoprotein. In the paraquat-induced lung injury model, exogenous surfactant tended to increase the survival rate and reduce pulmonary surface wall tension in rats. In humans, a commercial surfactant (survanta) may prove inadequate to treat patients with paraquat intoxication, as it does not include SP-D.
Supplementation with surfactant proteins may therefore prove efficacious in patients in the early stages of paraquat intoxication.
Our results demonstrate that there is a time-dependent variation in the levels of surfactant proteins after paraquat injury, and suggest that these proteins perform a function in the protection of lung tissue from ROS-induced injury.
References
1. Rose MS, Smith LL, Wyatt I. Evidence for energy-dependent accumulation of paraquat into rat lung. Nature 1974. 252:314–315PMID : 4431454.


2. Forman HJ, Aldrich TK, Posner MA, Fisher AB. Differential paraquat uptake and redox kinetics of rat granular pneumocytes and alveolar macrophages. J Pharmacol Exp Ther 1982. 221:428–433PMID : 7077538.

3. Winchester JF. In: Hadadad LM, Winchester JF, eds. Paraquat and the bipyridyl herbicides. Clinical management of poisoning and drug overdose 1990. 2nd ed. Philadelphia: WB Saunders, 1088–1103.
4. Manktelow BW. The loss of pulmonary surfactant in paraquat poisoning: a model of the study of the respiratory distress syndrome. Br J Exp Pathol 1967. 48:366–369PMID : 5182320.


5. Clark HW, Reid KB, Sim RB. Collectins and innate immunity in the lung. Microbes Infect 2000. 2:273–278PMID : 10758403.


6. LeVine AM, Whitsett JA. Pulmonary collectins and innate host defense of the lung. Microbes Infect 2001. 3:161–166PMID : 11251302.


7. Botas C, Poulain F, Akiyama J, Brown C, Allen L, Goerke J, Clements J, Carlson E, Gillespie AM, Epstein C, Hawgood S. Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D. Proc Natl Acad Sci U S A 1998. 95:11869–11874PMID : 9751757.



8. Wert SE, Yoshida M, LeVine AM, Ikegami M, Jones T, Ross GF, Fisher JH, Korfhagen TR, Whitsett JA. Increased metalloproteinase activity, oxidant production, and emphysema in surfactant protein D gene-inactivated mice. Proc Natl Acad Sci U S A 2000. 97:5972–5977PMID : 10801980.



9. Barrios R, Pardo A, Ramos C, Montano M, Ramirez R, Selman M. Upregulation of acidic fibroblast growth factor during development of experimental lung fibrosis. Am J Physiol 1997. 273:L451–L458PMID : 9277459.

10. Selman M, Montano M, Ramos C, Barrios R, Perez-Tamayo R. Experimental pulmonary fibrosis induced by paraquat plus oxygen in rats: a morphologic and biochemical sequential study. Exp Mol Pathol 1989. 50:147–166PMID : 2707380.


12. Fisher HK, Clements JA, Wright RR. Pulmonary effects of the herbicide paraquat studied 3 days after injection in rats. J Appl Physiol 1973. 35:268–273PMID : 4740607.

13. Fisher HK, Clements JA, Tierney DF, Wright RR. Pulmonary effects of paraquat in the first day after injection. Am J Physiol 1975. 228:1217–1223PMID : 236669.

14. Robertson B, Enhorning G, Ivemark B, Malmqvist E, Modee J. Experimental respiratory distress induced by paraquat. J Pathol 1971. 103:239–244PMID : 5208417.


15. Risberg B, Smith L, Ortenwall P. Oxygen radicals and lung injury. Acta Anaesthesiol Scand Suppl 1991. 95:106–118PMID : 1927220.


16. Smith LL. The toxicity of paraquat. Adverse Drug React Acute Poisoning Rev 1988. 7:1–17PMID : 3291571.


17. Tominack RL, Pond SM. In: Goldfrank LR, Flomenbaum NE, Lewin NA, Howland MA, Hoffman RS, Nelson LS, eds. Herbicide. Goldfrank's toxicologic emergencies 2002. 7th ed. USA: McGraw-Hill, 1393–1410.
18. Chen CM, Lua AC. Lung toxicity of paraquat in the rat. J Toxicol Environ Health A 2000. 60:477–487PMID : 12607909.


19. Nogee LM. Alterations in SP-B and SP-C expression in neonatal lung disease. Annu Rev Physiol 2004. 66:601–623PMID : 14977415.


20. Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 2005. 5:58–68PMID : 15630429.


21. Wright JR. Immunomodulatory functions of surfactant. Physiol Rev 1997. 77:931–962PMID : 9354809.


22. Cockshutt AM, Weitz J, Possmayer F. Pulmonary surfactant-associated protein A enhances the surface activity of lipid extract surfactant and reverses inhibition by blood proteins in vitro. Biochemistry 1990. 29:8424–8429PMID : 2252903.


23. Suzuki Y, Fujita Y, Kogishi K. Reconstitution of tubular myelin from synthetic lipids and proteins associated with pig pulmonary surfactant. Am Rev Respir Dis 1989. 140:75–81PMID : 2751175.


24. Fisher AB, Dodia C, Chander A. Inhibition of lung calcium-independent phospholipase A2 by surfactant protein A. Am J Physiol 1994. 267:L335–L341PMID : 7943260.

25. Touqui L, Arbibe L. A role for phospholipase A2 in ARDS pathogenesis. Mol Med Today 1999. 5:244–249PMID : 10366819.


26. Bridges JP, Davis HW, Damodarasamy M, Kuroki Y, Howles G, Hui DY, McCormack FX. Pulmonary surfactant proteins A and D are potent endogenous inhibitors of lipid peroxidation and oxidative cellular injury. J Biol Chem 2000. 275:38848–38855PMID : 10969075.


27. Clark H, Palaniyar N, Strong P, Edmondson J, Hawgood S, Reid KB. Surfactant protein D reduces alveolar macrophage apoptosis in vivo. J Immunol 2002. 169:2892–2899PMID : 12218102.


28. Pan T, Nielsen LD, Allen MJ, Shannon KM, Shannon JM, Selman M, Mason RJ. Serum SP-D is a marker of lung injury in rats. Am J Physiol Lung Cell Mol Physiol 2002. 282:L824–L832PMID : 11880309.


29. Gommers D, Eijking EP, So KL, vantVeen A, Lachmann B. Bronchoalveolar lavage with a diluted surfactant suspension prior to surfactant instillation improves the effectiveness of surfactant therapy in experimental acute respiratory distress syndrome (ARDS). Intensive Care Med 1998. 24:494–500PMID : 9660267.


Figure 1
Variations in the levels of surfactant protein D (SP-D) over time in rats treated with paraquat. SP-D was quantified via Western blot analysis and data were expressed as a fraction of that measured in the control group. Data are expressed as the meanSD values. No significant differences were detected in the initial SP-D levels among the groups (as assessed by ANOVA). The SP-D level in the 72-hour postinjection group was significantly higher than those of the control, and the 24-hour and 48-hour postinjection groups (* ; p<0.05) (Student's t-test).

Figure 2
Variations in the levels of surfactant protein A (SP-A) over time in rats treated with paraquat. SP-A was quantified via Western blot analysis and data are expressed as a fraction of that measured in the control group. Data are presented as the meanSD values. Differences between groups were statistically significant (as assessed by ANOVA): SP-A levels in the control, and 6-hour and 12-hour postinjection groups were significantly different from those of the 24-hour, 48-hour, and 72-hour groups (* ; p<0.05) (Tukey's test)
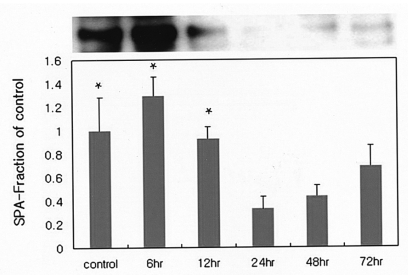
Figure 3
Variations in the levels of lipid peroxidation over time in rats treated with paraquat. Concentrations of thiobarbituric acid-reactive substances (TBA-RS) in left lung tissue, expressed as a fraction of that measured in the control group. Data are expressed as meanSD values. Differences between groups were statistically significant (as assessed by ANOVA): levels of lipid peroxidation in the control group differed significantly from those measured in the 6-hour postinjection group (* ; p<0.05) (Tukey's test)
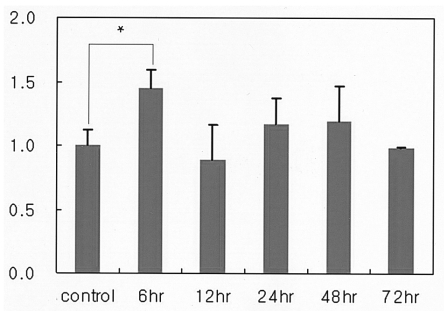
Figure 4
Light micrograph showing the inflammatory cell infiltration occurring after paraquat injection (hematoxylin and eosin, 100). The control (A) and 6-hour postinjection groups (B) evidence normal cytoarchitecture. The 12-hour postinjection group (C) exhibits inflammatory cell infiltration around the bronchiole. The 72-hour postinjection group (D) exhibits diffuse lung damage on the parenchyma and peribronchiole.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



