INTRODUCTION
Pneumatosis intestinalis (PI) is an uncommon disorder characterized by an accumulation of gas in the large or small bowel wall, and has been associated with a variety of disorders and procedures. Organ transplantation, bowel ischemia, inflammatory bowel disease, obstructive airway disease, infectious agents such as Clostridium difficile and cytomegalovirus, and the administrations of steroids or immunosuppressive agents are well known to predispose PI1). Although the etiology of PI remains unclear, a number of theories have been proposed, the two main ones being mechanical and bacterial2). According to the mechanical theory, gas dissects the abdominal wall forming the intestinal lumen or lungs; whereas according to the bacterial theory, bacteria enter the bowel wall and produce gas within the intestinal wall3).
Here, we describe a case of PI with pneumoperitoneum mimicking intestinal perforation after non-myeloablating stem cell transplantation, which responded successfully to medical treatment. Our experiences support the mechanical rather than the bacterial theory of the pathogenesis of PI.
Go to : 

CASE REPORT
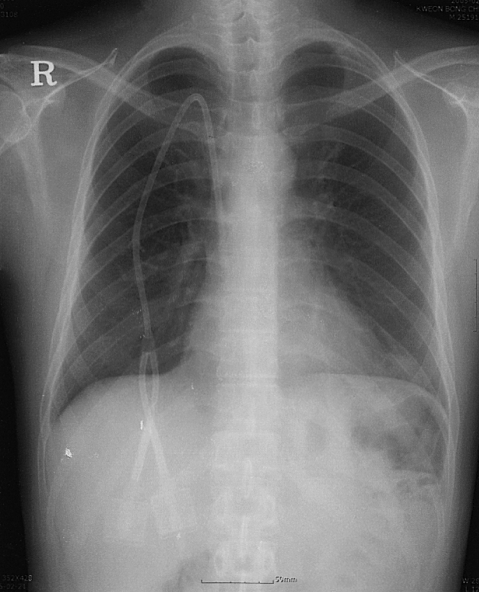
A 35-year-old man was admitted to hospital because of pneumoperitoneum, which was incidentally detected in a chest X-ray without any abdominal symptoms. The patient had been diagnosed as having myelodysplastic syndrome 5 years before admission, and had received regular follow-up with supportive care. During this follow-up, anemia had been slowly aggravated and a bone marrow biopsy showed an increase in blasts and refractory anemia with excess blast phase. Therefore, two months before admission, allogenic non-myeloablating stem cell transplantation was performed with a DQ mismatch donor. Five days post-transplantation, he developed diarrhea of more than ten times per day. Sigmoidoscopy performed at the time demonstrated submucosal hemorrhage and hyperemic mucosal change, and an endoscopic biopsy revealed graft-versus-host-disease grade 1. Accordingly, cyclosporine A (based on serum creatinine levels and monitored blood cyclosporine levels) and prednisolone (60mg daily) were prescribed. After immunosuppressive treatment, the diarrhea slightly, but persistently, improved. One month later, complete remission was obtained by follow up bone marrow biopsy and peripheral blood DNA examination of tandem repeat numbers.
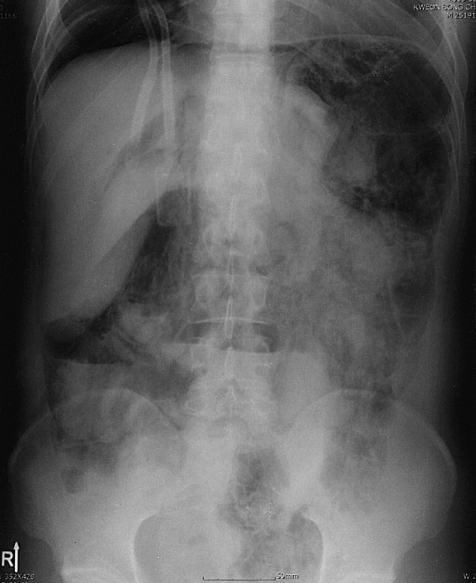
The patient returned to clinic for regular follow-up, and routinely underwent chest X ray. Just prior to admission, an abdominal plain film showed pneumoperitoneum and PI (Figure 1). However, he had no abdominal pain, discomfort, or fever, though he passed watery diarrhea ten times a day for one month, since his last hospitalization, which we presumed was a symptom of graft-versus-host-disease, as confirmed by sigmoidoscopic biopsy. At admission, his temperature was 36.5℃, pulse rate 80/minute, respiration 20/min, and blood pressure 100/70 mmHg. A physical examination revealed no specific findings. The abdominal wall was soft and flat, bowel sounds were normoactive, and tenderness and rebound tenderness were absent. A CT scan of the abdomen and pelvis obtained with radiocontrast dye, showed free air in peritoneum and intramural gas in whole colon (Figure 2). The colonic wall was thickened from sigmoid to cecum, but the small bowel and mesentery were relatively intact. No portal venous gas was observed, and the peritoneal enhancing pattern was not prominent. A CT scan showed retroperitoneal gas and pneumomediastinum as well as pneumopericardium. There was no evidence of leukocytosis, and CRP was not elevated.
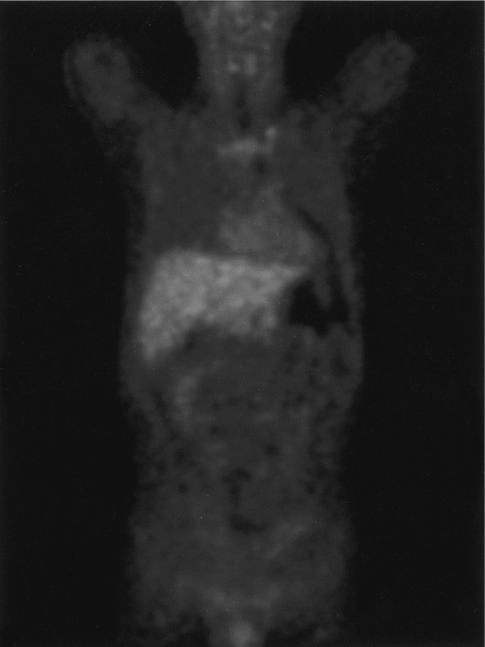
Bowel rest and antibiotic therapy consisting of cefotaxime, amikacin, vancomycin and metronidazole, targeting gram negative bacteria, anaerobes and Clostridium species, were started. PET-CT was used to confirm peritoneal or abdominal inflammation (Figure 3). However, work-up failed to demonstrate peritoneal inflammation or an infectious etiology. During hospitalization, he had a sense of well- being, and follow up X-rays showed spontaneous resolution of free and intramural air. After two weeks of antibiotic therapy, he commenced feeding by mouth and was discharged with radiological improvement (Figure 4).
Go to : 

DISCUSSION
Nowadays, because hematopoietic stem cell transplantation has become one of the most effective treatment modalities for many hematologic malignancies, abdominal complications in post-transplanted patients have become more important. Most abdominal complications are due to gastrointestinal infections, and some of these infectious agents, such as Clostridium difficile and cytomegalovirus, can induce PI4).
Clinical manifestations of PI vary, and range from an incidental finding that is invariably clinically benign, to a severe life threatening form5). Therefore, PI is considered a spectrum of secondary findings caused by underlying diseases, and its severity is determined by interactions between mucosal integrity, intraluminal pressure, bacterial flora, and intraluminal gas1).
In the described case, there was no clear evidence of peritonitis; the patient had no abdominal pain or rebound tenderness. Furthermore, no abnormal uptake was present on PET-CT images obtained to confirm peritoneal inflammation. We presumed that no direct communication was present between lumen and peritoneum, and thus planned medical treatment rather than emergency surgery. In the event, PI improved spontaneously after two weeks of bowel rest and antibiotic therapy.
The pathogenesis of PI is unclear, as is the origin of the intramural or intraperitoneal gas. The two main theories propose mechanical and bacterial bases for the pathogenesis of PI. According to the mechanical theory, increased intraluminal pressure, caused by numerous conditions like gastrointestinal obstruction, causes gas to diffuse into, and dissect, the bowel wall from intestinal lumen1). Enhanced mucosal permeability to gas or mucosal damage, such as that caused by blunt trauma, accelerates bowel wall dissection and cystic gas formation. Moreover, steroids are known to alter mucosal permeability and the gut immune barrier. Steroids can shrink Peyer's patches, which results in a loss of structural integrity in mucosa and submucosa, allowing gas to enter into the mucosa6, 7).
On the other hand, the bacterial theory proposes that gas-forming bacteria invade the bowel wall and produce intramural gas by fermentation. The intramural injection of Clostridium difficile has been shown to initiate PI formation in rat animal model8). This theory is not adequately supported by bacteriologic identification or by the presence of inflammations in surgically obtained tissues9).
In addition to these two theories, a pulmonary gas theory has been proposed based on the association between pulmonary disease and PI10). According to this theory, alveolar rupture could result in the dissection of air along vascular channels in the mediastinum, tracking caudally to the retroperitoneum and then to the bowel mesentery. Furthermore, intermittent elevations of intra-abdominal pressure during coughing result in intraluminal pressure fluctuations, which drive transmural gas dissection.
In the described case, the patient had many well-known risk factors for PI, including transplantation, immunosuppressive agent administration, and sigmoidoscopy. This case supports the mechanical theory; free air in the abdomen probably represents the dissection of sterile intramural gas through the serosal surface of the intestine without causing through bowel wall perforation11-14). We are of this opinion because peritoneal irritation symptoms and signs induced by direct communications between peritoneum and septic lumen of bowel were absent in this case, and because there was no evidence of bacterial infection.
PI may be treated by conservatively or surgically, though indications for surgical intervention have not been agreed. In many reports, patients with pneumoperitoneum have undergone surgery, because abdominal free air was regarded as bowel perforation, although no hollow organ perforation was found in some cases15, 16).
Overall reported mortality rates vary from 0% to 75%5, 17-19), but mortality is highly dependent on coexisting conditions, such as, bowel ischemia or inflammation. Wiesner et al.19) analyzed 23 PI patients, and found that 16 patients showed portomesenteric venous gas; overall PI associated mortality was 44%. However, in patients who showed portomesenteric venous gas and PI on CT scans, mortality increased to 72%. The presence of portomesenteric gas is probably associated with a higher mortality rate, because CT findings of portomesenteric gas usually indicate the presence of mesenteric infarct or ischemia. Range of transmural infarction can also affect mortality, which has been reported to increase with the number of involved segments19).
Another considerable factor is serum bicarbonate. According to a report by Kurbegov20), mean serum bicarbonate concentration is significantly lower in patients with a poor outcome group in PI. Low serum bicarbonate may be caused by necrotic bowel tissue, and represents metabolic acidosis. It results in a poor prognosis and requires surgical intervention or will cause death in PI.
To determine the need for surgical intervention, it is most important to establish the presence of poor prognosis factors associated with mortality. Moreover, the following should be considered; 1) bowel ischemia or necrosis, 2) portomesenteric venous gas by CT, 3) metabolic acidosis, 4) severity of PI extent, and 5) sepsis of an abdominally originated infection. If symptoms or signs compatible with the above factors are present, early surgical intervention should be performed as soon as possible to avoid life threatening complications resulting from an unremarkable bowel perforation. However, not all pneumoperitoneum PI cases require surgery.
Of the many factors that affect the GI tract, mucosal integrity, intramural pressure, bacterial flora, and intraluminal gas interact to form PI1). Moreover, it is not known whether PI is a primary or secondary event. PI is a disease spectrum that ranges from benign to highly aggressive patterns that depend on patient underlying condition. However, no discernable feature has been identified that differentiates these two extreme patterns of PI. Nevertheless, many predictive factors indicate whether PI is likely follow a medically treatable benign course or an aggressive course requiring surgical intervention.
In conclusion, we report a case of PI with free air mimicking intestinal perforation, which responded successfully to conservative non-operative management. Our report supports the mechanical theory of the pathogenesis of PI. In addition, we reviewed indications for surgical intervention and the status of factors associated with a poor outcome.
Go to : 





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





