 |
 |
| Korean J Intern Med > Volume 21(4); 2006 > Article |
|
Abstract
Lung cancer metastases can occur in almost any organ. However, metastasis of small cell lung cancer to the pancreas is rare. Moreover, not all cases present with clinically diagnosed pancreatitis. We recently treated a patient with small cell lung carcinoma that invaded the pancreatic duct causing acute pancreatitis. Generally, the treatment for tumor-induced acute pancreatitis is initially supportive followed by aggressive chemotherapy or surgery. If the patient can tolerate the insertion of an endoscopic intrapancreatic stent, this is performed in addition to chemotherapy and surgery; this approach offers a safe and effective treatment modality for such patients.
Lung cancer affects approximately 26.4/100,000 people per year and is one of the leading causes of cancer related death in Korea and Western countries1). Lung cancer metastases can occur in any organ and manifests as a variety of clinical problems. The common sites of metastasis from lung cancer include the regional lymph nodes, brain, bone, adrenal gland and lung. Among the many types, small cell lung cancer (SCLC) is an aggressive malignancy with a high propensity for early regional and distant metastasis2). Acute pancreatitis caused by cancer metastases is quite unusual. We recently treated a patient with acute pancreatitis caused by small cell lung carcinoma invading the pancreatic duct. We here describe this case treated with endoscopic therapy and review previously reported cases to better understand the clinical features and improve the treatment of this rare condition.
A 45-year-old female was admitted to the Kyung Hee Medical Center in April 2005 with a 20-day history of gnawing epigastric pain radiating to the back. In November 2001, she visited our hospital complaining of a chronic cough. Bronchoscopic washing cytology results revealed a SCLC in the left lower lung field, which was treated with chemotherapy and radiation therapy. It appeared that the SCLC responded to the therapy at that time, but follow-up computed tomography (CT) showed a new mass at the right lower lung field. A biopsy revealed that the SCLC had relapsed and she was admitted for additional chemotherapy treatments. Unfortunately, she did not respond to therapy, and a right lung lobectomy was performed. In July 2004, a submandibular mass was found incidentally, and a biopsy indicated metastatic SCLC. Another chemotherapy treatment was started. One month later, PET/CT revealed a left adrenal gland mass suggestive of a metastatic lesion. Additional chemotherapy regimens were provided with no improved response. In January 2005, chemotherapy was discontinued and the patient received only supportive care.
The patient had a history of heavy drinking and a 15-pack-year smoking history. However, she had stopped drinking and smoking during the last four years. In addition, she was not taking any other medication. She appeared acutely ill and dehydrated. On physical examination, the patient's body temperature was 38.5Ōäā, blood pressure was 120/80 mmHg, her pulse was 78/minute and her respiratory rate was 20/minute. The breath sounds were decreased in the right lung field. Mild tenderness was noted at the epigastrium but there was no palpable mass. The initial laboratory data were as follows: WBC 6,710/mm3 (82.1% neutrophils, 9.6% lymphocytes, and 4.5% monocytes), hematocrit 36.3%, platelets 170,000/mm3, glucose 110 mg/dL, BUN 7 mg/dL, creatinine 0.6 mg/dL, sodium, 139 mmol/L, potassium 3.9 mmol/L, and calcium 8.4 mg/dL. Serum AST 27 IU/L, serum ALT 15 IU/L, serum gamma glutamyl transpeptidase 13 IU/L, serum alkaline phosphatase 66 IU/L, and amylase 2,650 U/L (normal, 25 to 125 U/L). The chest X-ray revealed the right lower lobectomy and lymph node calcifications at the anterior posterior window area. The abdominal CT revealed multiple low attenuated masses in the pancreatic head, a dilated upstream pancreatic duct and a left adrenal metastasis (6.8 ├Ś 5.3 cm) (Figure 1). No peripancreatic infiltration or fluid collection was noted. Endoscopic ultrasonography (EUS) showed several, well demarcated, irregular hypoechoic masses at the head portion of the pancreas, which compressed the pancreatic duct resulting in a dilatation of the upstream pancreatic duct (Figure 2). The patient was initially treated with intravenous fluids, dietary restrictions and intravenous analgesics. However, her pain continued unabated. Therefore, endoscopic retrograde cholangiopancreatography (ERCP) was performed to relieve the pancreatic ductal pressure. The ERCP (JF-240, Olympus, Tokyo, Japan) showed an irregular stricture of the pancreatic duct at the head portion of the pancreas along with mild dilatation of the upstream pancreatic duct (Figure 3). Accordingly, a naso-pancreatic drainage tube (5 Fr, Wilson-Cook, USA) was inserted across the stricture up to the tail of the pancreatic duct. After the pancreatic drainage procedure, the patient's epigastric pain dramatically improved. In addition, the serum amylase and lipase level decreased to 189 U/L and to 143 U/L respectively, one day after the pancreatic drainage procedure. On the sixth day of hospitalization, a second ERCP was done to replace the naso-pancreatic drainage tube with a plastic pancreatic stent. However, selective cannulation of the pancreatic duct was impossible due to the subcutaneous edema of the major papilla caused by incorrect injection of the dye used. Fortunately, the patient did not complain of any discomfort for several days, even after eating regular meals. The patient was discharged without any problems.
A few days later the patient was readmitted with epigastric pain. On physical examination, she presented with epigastric tenderness and equivocal rebound tenderness. The serum amylase level was 1,867 U/L. Recurrent acute pancreatitis due to a pancreatic metastasis was suspected. An emergency ERCP was performed. The pancreatography showed a short stenosis of the main pancreatic duct, which was identical to the portion found during the first ERCP. A 5 Fr pancreatic stent, 9 cm in length, was inserted at the pancreatic duct through the stenotic area (Figure 3). After inserting the stent, the patient's abdominal pain continued to decrease and she could take food po; she was discharged without problems.
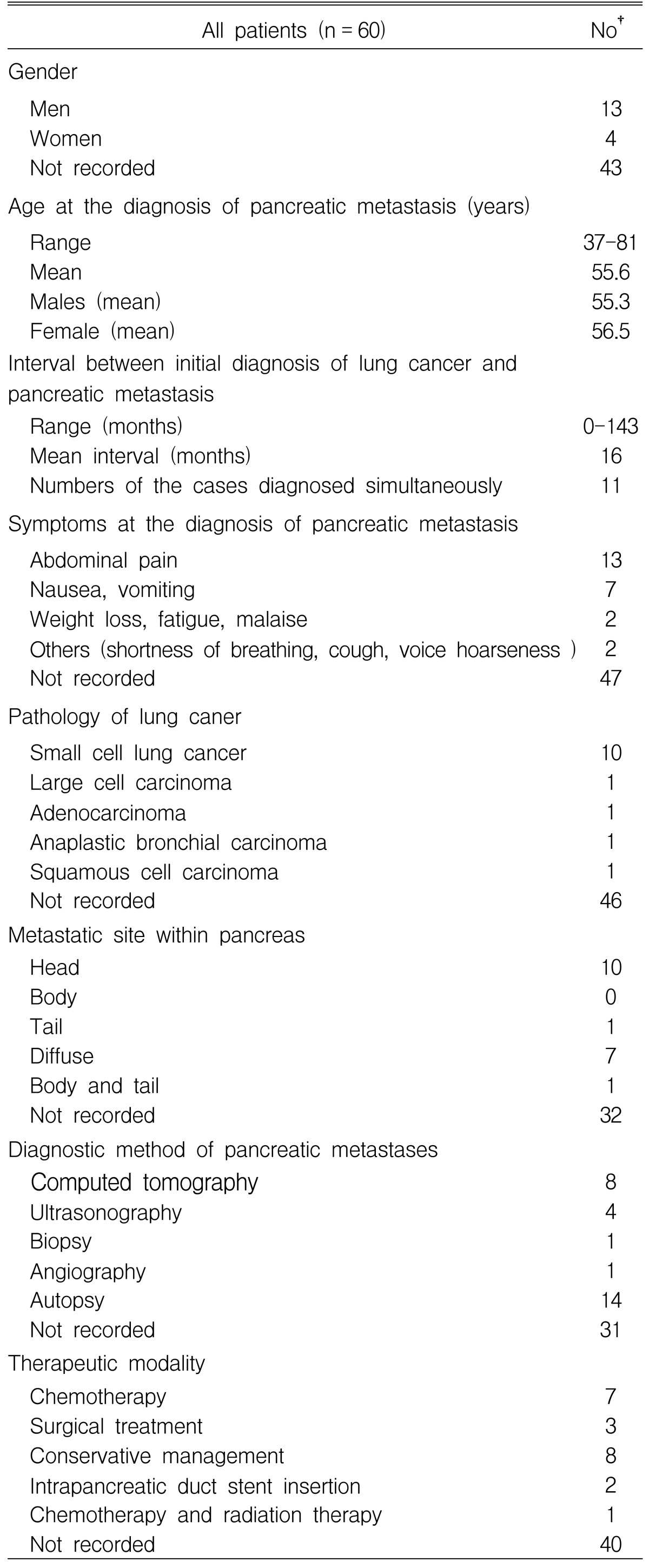
Metastatic tumors of the pancreas are uncommon. The incidence of metastases to the pancreas has been reported to vary from 3 to 10.6% at autopsy in patients with a malignant neoplasm3). A variety of tumors have been reported to metastasize to the pancreas including: renal cell carcinoma, prostate, breast, colon, melanoma, Hodgkin's disease and carcinoma of the lung4). Minni et al. reviewed 333 cases of pancreatic metastases3). The primary origins of the metastases were reported to be as follows: 150 (45%) from a renal cell carcinoma, 49 (14.7%) from a lung carcinoma, 25 (7.5%) from a breast carcinoma and 22 (6.6%) from colon cancer. In addition, 60 cases, in which a lung carcinoma had metastasized to the pancreas, were reviewed3-12). As shown in Table 1, the most common histological type of metastatic lung carcinoma to the pancreas is the small cell carcinoma, followed by large cell carcinoma, squamous cell carcinoma and anaplastic bronchial carcinoma. This is because a small cell carcinoma of the lung is characterized by diffuse metastases by lymphatic and hematogenous spread2).
Not all cases of pancreatic metastases present with clinical pancreatitis. Metastatic invasion to the pancreas is a rare cause of acute pancreatitis. Typically, it occurs in patients known to have advanced carcinoma. Among the reported cases of metastatic lung carcinoma to the pancreas (Table 1), 13 patients had acute pancreatitis. These cases are summarized in Table 24-12). The suggested mechanisms included: 11 (84%) due to mechanical ductal obstruction and/or rupture in the pancreas, and two (16%) due to compression from an enlarged peripancreatic lymph node. Other causes cited in the literature include rupture or vascular compromise due to tumor invasion12). In our patient, the cause of the pancreatitis was initially suspected to be a pancreatic duct obstruction as a result of the intrapancreatic metastatic mass or by a direct pancreatic invasion of the metastatic adrenal mass. However, EUS and ERCP showed the intrapancreatic metastatic mass to be a compressed pancreatic duct. Furthermore, the patient responded to the pancreatic drainage procedure. Therefore, the etiology of the acute pancreatitis in our patient was mechanical duct obstruction.
There are several reports suggesting the diagnostic tools that are essential for making a proper diagnosis of a pancreatic metastasis. On ultrasound study, the metastases appear in the pancreatic parenchyma as solid, space-occupying lesions with internal structure more hypoechoic than normal pancreatic tissue7). The spiral CT generally shows a solitary mass or multiple small pancreatic nodules appearing as well defined hyper-enhancing tumors that can be distinguished from the normal pancreatic parenchyma. Hyper enhancement of pancreatic metastases results from their hypervascular pattern relative to normal pancreatic parenchyma; this finding serves not only to aid in the detection of the tumor but also to distinguish it from a primary adenocarcinoma of the pancreas13). EUS is the most accurate diagnostic modality for evaluating the pancreas. For tumors < 2 cm in diameter, the detection rate of EUS is as high as 100%, which is in contrast to the 60% and 50% obtained with US and CT, respectively, with the exception of a carcinoma in situ14). The EUS features of primary pancreatic cancer and metastatic lesions are similar with respect to the tumor size, echogenicity and consistency. However, metastatic tumors are more likely to have well defined borders compared with primary tumors15). Therefore, EUS visualization of a well-defined pancreatic mass, in a patient with a history of malignancy, should increase suspicion of a metastatic lesion. In our case, well defined masses were identified at the head of the pancreas; this compressed the pancreatic duct and resulted in dilatation of the upstream pancreatic duct observed by the EUS. The pattern of ductal changes shown by ERCP can also provide good diagnostic information. ERCP can be performed in these settings except in cases where the general patient condition is poor. ERCP can be used to confirm the degree of stenosis and irregularity of the pancreatic duct and therefore provide a more accurate assessment. A tissue biopsy should be performed in order to confirm the diagnosis of metastases induced pancreatitis15, 16). DeWitt et al. reported that EUSFNAB provided an initial diagnosis of malignancy, tumor recurrence and pancreatic metastases15). However, EUS FNAB was not performed in our case because the equipment was unavailable.
Traditionally, treatment for tumor induced acute pancreatitis is initially supportive; however, in our case conservative therapy for this rare clinical problem was unsuccessful (Table 2). Aggressive chemotherapy has been advocated to hasten the resolution of acute pancreatitis in patients with small call carcinoma9). In our case, conservative management with dietary restriction and intravenous fluid was initially attempted, but there was no improvement. In addition, several attempted chemotherapy regimens failed. The patient's medical condition allowed for an ERCP to be performed, and for a stent to be inserted into the intrapancreatic duct via the ERCP. Subsequently, her pain subsided and the laboratory findings were dramatically improved.
In 1999, Kim et al. reported a metastasis-induced acute pancreatitis in a patient with small cell carcinoma of the lung11). This was the first case of a small cell carcinoma of the lung presenting with acute pancreatitis reported in Korea. However, in this report, the acute pancreatitis occurred during chemotherapy. Therefore, the possibility that the acute pancreatitis was due to chemotherapeutic agents could not be excluded. On the other hand, our case was unique in two ways. First, the patient had not taken any medications during the three months before the onset of acute pancreatitis; second, she was treated with endoscopic intrapancreatic stenting. This is the first report of this therapeutic approach in Korea. Only a few cases have been reported previously in the medical literature.
In conclusion, this case demonstrates that acute pancreatitis can occur as a manifestation of a metastasis of small cell lung cancer. Furthermore, stenting through ERCP offers a safe and effective alternative treatment modality in addition to chemotherapy and surgical treatment.
References
1. National Statistical Office. Annual report on the cause of death statistics. 2003;Republic of Korea.
2. DeVita VT, Hellman S, Rosenberg SA. Cancer: principles and practice of oncology. 2005;7th ed. Philadelphia: Lippincott Williams & Wilkins Inc, 810ŌĆō843.
3. Minni F, Casadei R, Perenze B, Greco VM, Marrano N, Margiotta A, Marrano D. Pancreatic metastases: observations of three cases and review of the literature. Pancreatology 2004;4:509ŌĆō520PMID : 15316227.


4. Niccolini DG, Graham JH, Banks PA. Tumor-induced acute pancreatitis. Gastroenterology 1976;71:142ŌĆō145PMID : 1278639.


5. Yeung KY, Haidak DJ, Brown JA, Anderson D. Metastasis-induced acute pancreatitis in small cell bronchogenic carcinoma. Arch Intern Med 1979;139:552ŌĆō554PMID : 220925.


6. Noseda A, Gangji D, Cremer M. Acute pancreatitis as presenting symptom and sole manifestation of small cell lung carcinoma. Dig Dis Sci 1987;32:327ŌĆō331PMID : 3028735.


7. Wernecke K, Peters PE, Galanski M. Pancreatic metastases: US evaluation. Radiology 1986;160:399ŌĆō402PMID : 3523591.


8. Chowhan NM, Madajewicz S. Management of metastases-induced acute pancreatitis in small cell carcinoma of the lung. Cancer 1990;65:1445ŌĆō1448PMID : 2155057.


9. Stewart KC, Dickout WJ, Urschel JD. Metastasis-induced acute pancreatitis as the initial manifestation of bronchogenic carcinoma. Chest 1993;104:98ŌĆō100PMID : 8391965.


10. Gutman M, Inbar M, Klausner JM. Metastases-induced acute pancreatitis: a rare presentation of cancer. Eur J Surg Oncol 1993;19:302ŌĆō304PMID : 8390948.

11. Kim KH, Kim CD, Lee SJ, Lee G, Jeen YT, Lee HS, Chun HJ, Song CW, Um SH, Lee SW, Choi JH, Ryu HS, Hyun JH. Metastasis-induced acute pancreatitis in a patient with small cell carcinoma of the lung. J Korean Med Sci 1999;14:107ŌĆō109PMID : 10102535.



12. Papagiannis A, Zarogoulidis K, Delis D, Patakas D. A 52-year-old man with a lung mass and acute abdominal pain. Chest 2000;117:894ŌĆō896PMID : 10713023.


13. Maeno T, Satoh H, Ishikawa H, Yamashita YT, Naito T, Fujiwara M, Kamma H, Ohtsuka M, Hasegawa S. Patterns of pancreatic metastasis from lung cancer. Anticancer Res 1998;18:2881ŌĆō2884PMID : 9713480.

14. Maguchi H. The roles of endoscopic ultrasonography in the diagnosis of pancreatic tumors. J Hepatobiliary Pancreat Surg 2004;11:1ŌĆō3PMID : 15747027.


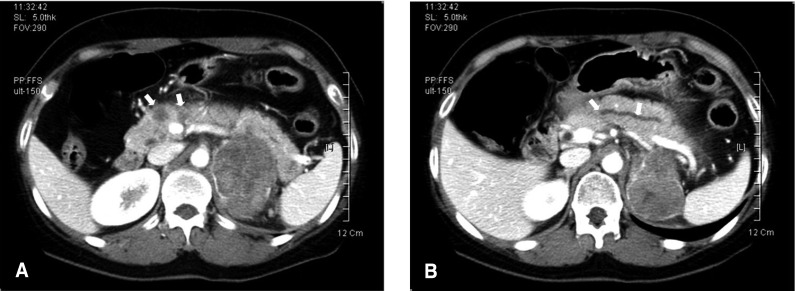
Figure┬Ā1
Computed tomography of the abdomen shows multiple low attenuated masses (arrows) at the pancreatic head (A) and a dilatation of the upstream pancreatic duct (arrows) (B). CT also reveals a left adrenal mass of 6.8 ├Ś 5.3 cm.
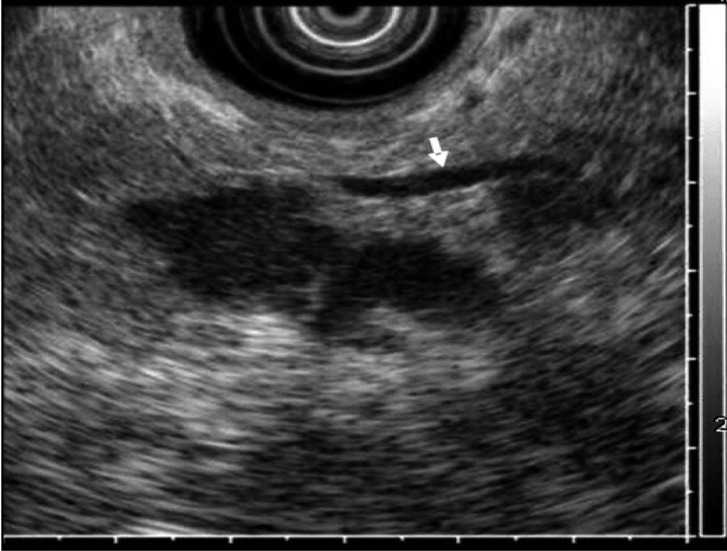
Figure┬Ā2
Endoscopic ultrasonography reveals several, well demarcated, irregular hypoechoic masses at the head portion of the pancreas, which compressed the pancreatic duct (arrow).
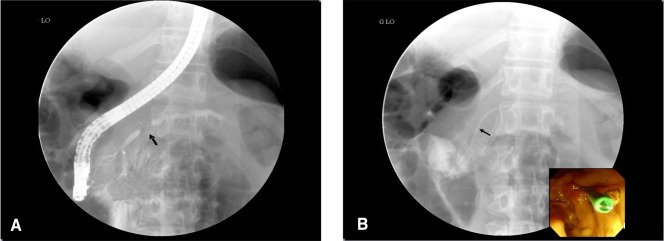
Figure┬Ā3
Endoscopic retrograde pancreatography reveals an irregular stricture of the pancreatic duct (arrow) at the head portion of the pancreas, along with mild dilatation of the upstream pancreatic duct (A). A 5 Fr pancreatic stent (arrow), 9 cm in length, was inserted at the pancreatic duct through the stenotic area (B).
Table┬Ā2
Reviewed cases with acute pancreatitis due to metastatic lung carcinoma.
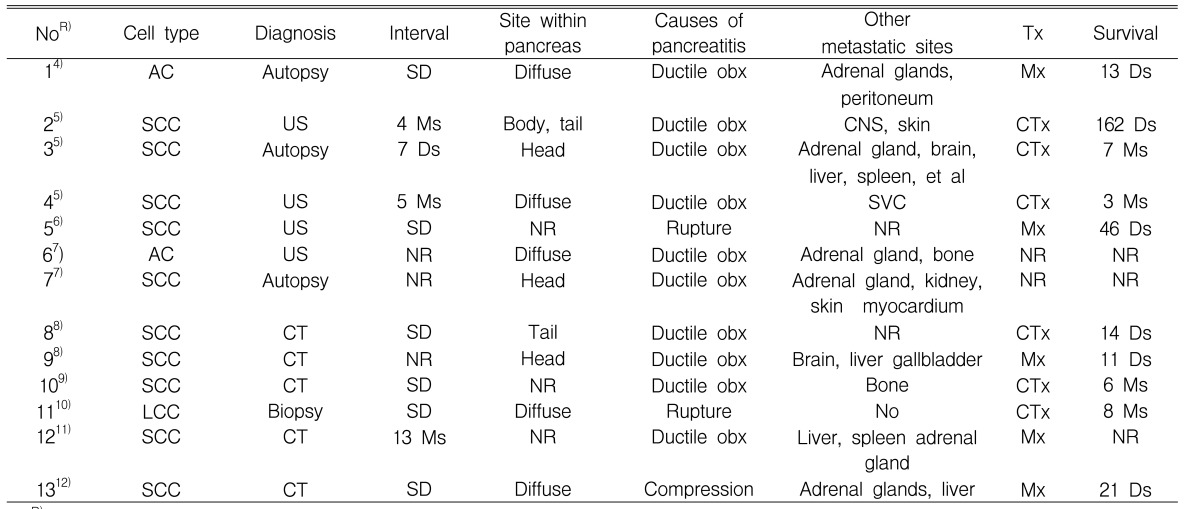
NoR), case number with reference; SCC, small cell carcinoma; LCC, large cell carcinoma; AC, anaplastic bronchial carcinoma; NR, not recorded; Interval, interval between initial diagnosis of lung cancer and pancreatic metastasis, Ms, months; Ds, days; SD, simultaneous detected; Ductile obx, mechanical ductal obstruction; Rupture, rupture in the pancreas; Compression, compression from enlarged peripancreatic lymph node; SVC, superior vena cava; Tx, Treatment; Mx, Conservative management, CTx, chemotherapy
-
METRICS

- Related articles
-
Non-small Cell Lung Cancer Initially Presenting with Intracardiac Metastasis2005 March;20(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


