 |
 |
| Korean J Intern Med > Volume 20(4); 2005 > Article |
|
Abstract
Background
The interindividual variability for the development of collaterals in coronary artery disease is dependent on the hypoxic induction level of VEGF. To determine whether the hypoxic induction of VEGF is controlled by the transcription of HIF-1 (alpha), the VEGF and HIF-1 (alpha) m-RNA levels were correlated to hypoxia in monocytes harvested from patients with coronary artery disease.
Methods
The collateral scoring system used was modified from the TIMI system. The mononuclear cell layer of the patients' blood was cultured in hypoxia (1% O2, 5% CO2, 94%N2) and normoxia (5% CO2, 95% room air) for 17 hours. The VEGF and HIF-1 (alpha) mRNA levels were measured using a RT-PCR technique. We calculated the fold inductionsof VEGF, HIF-1 (alpha) mRNA with hypoxia by dividing thehypoxic and the normoxic values.
Results
We found significantly higher hypoxic inductions of VEGF m-RNA in patients with collaterals compared to patients with no collaterals. However, there was no differencein the hypoxic inductions of HIF-1 (alpha) between the two groups (VEGF m-RNA mean fold inductions 3.71┬▒3.30 versus 1.65┬▒0.62, p=0.012, HIF-1(alpha) mRNA 1.42┬▒0.58 versus 1.20┬▒0.39, p=0.165).
Conclusions
We concluded that the interindividual variability in the hypoxic inductions of VEGF m-RNA in monocytes in patients is not controlled by the transcriptional levels of HIF-1 (alpha) with hypoxia. These findings suggest that a mechanism such as the post-transcriptional modification of HIF-1(alpha) is involved in the hypoxic inductions of VEGF.
VEGF is considered to play a major role in stimulating angiogenesis in various organs. Thus, it is a good candidate to stimulate neovascularization for limiting ischemic damage to the heart. Despite the unclear regulatory mechanism for VEGF, it is known that HIF-1 acts on the specific domain of the promoter region in VEGF1). Previous studies have reported that HIF-1 increases the VEGF levels in cardiac muscle cells when cultured under hypoxic conditions2).
Thus, it is considered likely that an elevated HIF-1 level is at least partially involved in VEGF expression in cardiac ischemia or myocardial infarction. (HIF-1 is a heterodimer with a basic helix-loop-helix structure consisting of an - and -subunit, a transcription factor expressed in low intracellular PO2.) HIF-1 is known to be involved in angiogenesis, glycolysis and erythropoiesis. Its target genes consist of VEGF, inducible nitric oxide synthase, lactate dehydrogenase, erythropoietin, glucose transporter and glycolytic enzyme3-6).
On the other hand, some animal studies have reported that the HIF-1 (alpha) mRNA levels were elevated in the retina or lungs when exposed to hypoxic conditions for some time, or conversely in cells cultured under hypoxic conditions7). However, the exact mechanism of HIF-1 regulation and the interaction of VEGF with target genes are as yet uncertain. The recruitment of monocytes is thought be an important step during collateral formation, second only to regional myocardial ischemia8). Increased shear stress in preformed epicardial collaterals is somewhat distant to the actual area of hypoxia and ischemia. This paves the way for monocyte accumulation, their maturation to macrophages, and the release of growth factors, all of which creates an inflammatory environment. In contrast to true angiogenesis, the process of arteriogenesis, which describes the growth of collateral vessels9), seems to be critically dependent on monocytes and on proper monocyte functions8).
Thus, we have assumed that individual differences in collateral circulation formation are affected by the VEGF expressed under hypoxic conditions. Additionally, the VEGF levels are regulated by the HIF-1 (alpha) mRNA level. To test this hypothesis, we cultured monocytes that were extracted from two patient groups (the collateral circulation group and the non-collateral circulation group) with significant lesions on coronary angiography. We compared the HIF-1 (alpha) mRNA levels to clarify the role of VEGF in the collateral circulation and to determine its correlation with HIF-1 (alpha).
Forty patients with coronary artery disease were included in this study. Each patient had at leasta 90% narrowing in more than one major coronary artery (except acute myocardial infarction). All patients gave their informed consent. The patients were recruited from those undergoing diagnostic coronary artery catheterization. The indications for catheterization for all patients were the presence of stable or unstable angina pectoris, or a suspected significant myocardial ischemia. Only patients with at least Ōēź1 coronary stenosis of Ōēź 90% (by visual analysis of the angiogram) and had no previous medication history were included in this study. The exclusion criteria were the presence of anemia, myocardial infarction, chronic inflammatory illness and neoplastic disease.
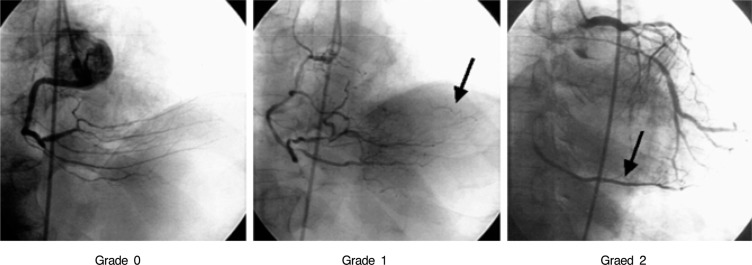
For each patient, the following data was collected and recorded via interview: name, registration number, gender, age, previous revascularizations, a familial history of cardiovascular diseases, and a medical history of diabetes mellitus, hypertension, hypercholesterolemia, and smoking history. Two physicians, experienced with coronary angiography, recorded the cardiovascular anatomy and the presence of collateral circulation after consulting with each other. They used a modified TIMI system to measure the degree of collateral circulation formation. Grade 0 referred to the absence of collateral circulation, grade 1 referred to an invisible blood stream in a recipient epicardial vessel, and grade 2 referred to a partial or complete filling of the recipient epicardial vessels by collaterals (Figure 1).
The mononuclear cell layer was isolated from peripheral blood using a mixture of polysaccharide and radiopaque contrast medium. During the coronary angiography, 40 mL of blood was collected from the femoral venous catheter, placed for catheterization before the angiography procedure began. The blood was immediately placed in a 50-mL polypropylene heparinized tube and kept on ice until it was used for monocyte isolation. After layering 20 mL of heparinized blood into a 15 mL tube containing Ficoll-Paque, it was centrifuged at 1,800 rpm at 18~20Ōäā for 30 minutes. From the upper layer in each tube, 8 mL of serum was collected and stored at 4Ōäā for later use. The middle phase, including the monocytes, was isolated and transferred to a 15 mL polypropylene centrifuge tube. The isolated monocyte layer was rinsed twice with a sterilephosphatebufferedsaline, then transferred to a centrifuge tube and centrifuged at 800 rpm at 18~20Ōäā for 10 minutes. After that, the supernatant was removed. The cell pellets were resuspended with 2 mL of Dulbecco's modifi edeagle's medium and a mixture of 2% fetal bovine serum and antibiotics. These included GIBCOBRL, high glucose powder with L-glutamine, 25 mM HEPES buffer with sodium chloride 4,750 mg/L replaces 6,400 mg/L with pyridoxine hydrochloride without sodium pyruvate without sodium bicarbonate. After sorting the cells by size and positioning them into tissue culture dishes (Corning), they were cultured in an incubator (Forma) at 95% room air and 5% CO2 at 37Ōäā for 1 hour, in order to induce monocyte adhesion. After confirming the monocyte adhesion, the culture medium was removed. Cells were replaced by a culture medium containing an 8 mL serum collected from the patients and cultured in a Triple Gas Incubator (Jouan. One dish contained 21% O2 and 5% CO2 and the other dish contained 1% O2, 5% CO2 and 94% N2. After culturing the cells under normal and hypoxic conditions for 17 hours, the RNA was extracted.
The cells, obtained under normal and hypoxic conditions, were treated with 1 mL RNAzolŌäóB (Biotec Laboratories Inc, Houston, Texas) in each sample and they were exposed to ice for 5 minutes. After that, the cells were centrifuged at 14,000 rpm for 15 minutes. The supernatant was transferred to a new tube and treated with isopropanol of an equivalent concentration in an ice pan for 10 minutes. This was followed by centrifugation at 12,000 rpm for 15 minutes. After centrifugation, the supernatant was removed, and the pellet was rinsed with 70% ethanol and then dried in air. DEPC distilled water was applied to the pellet and it was heated to dissolve at 60Ōäā for 30 minutes. The RNA was quantified using a spectrophotometer at a wavelength A260.
In 20 ┬ĄL reaction bowel, the following substances were mixed: 1 ┬Ąg RNA, 5 mM MgCl2, 1 ├Ś RNA PCR buffer, RNase-free distilled water, 1 mM dNTP Mixture, 1 units/mL RNase inhibitor, 0.25 units/L Reverse Transcriptase, and 2.5 ┬ĄL Random 9mer (TaKaRa, Tokyo, Japan). After that, the above substances were allowed to react at 30Ōäā for 10 minutes, at 42Ōäā for 30 minutes, at 99Ōäā for 5 minutes and at 5Ōäā for 5 minutes to prepare the cDNA. The product was then stored at 4Ōäā before it was used. In a mixture of 4 ┬ĄL cDNA, 1 ├Ś PCR buffer (50 mM KCL, 10 mL Tris-Cl [pH = 8.0]), 200 ┬ĄM dNTPs and 1 units of Taq DNA polymerase (TaKaRa, Tokyo, Japan), 20 pmol of primer was added to prepare 20 ┬ĄL in total, followed by PCR using a DNA thermal cycler. The PCR procedure consisted of the following three cycles: denaturation at 94Ōäā for 5 minutes, annealing at 60 - 66Ōäā for 1 minute and extension at 72Ōäā for 10 minutes.
The PCR product was treated onto 2% agarose gel. Electrophoresis was performed and the band was stained with ethidium bromide for observation under ultraviolet light. The PCR band was measured by densitometer. The VEGF and HIF-1 (alpha) mRNA levels were measured using a RT-PCR procedure consisting of 30 cycle and an annealing temperature of 60Ōäā/1min for VEGF, 65Ōäā/1min for HIF-1 (alpha). Each primer sequences were recorded in Table 1. The amount of calculatedfold inductions of VEGF and HIF-1 (alpha) mRNA for the hypoxic condition was done by dividing the hypoxic product value by the normoxic product value. The procedure was repeated three times and then averaged.
All data were described as means┬▒SD (SD: standard deviation). The data were tested with Student's t-test and Chi-square tests. The three collateral circulation groups (VEGF and HIF-1 (alpha) mRNA) were compared using analysis of variance (ANOVA) and Tukey's test for multiple comparisons. A p value<0.05 was considered as significant.
The statistical analysis was performed using SPSS-PC10.0 (Statistical package for the Social Sciences, SPSS Inc. Chicago, IL, USA) for MS Windows.
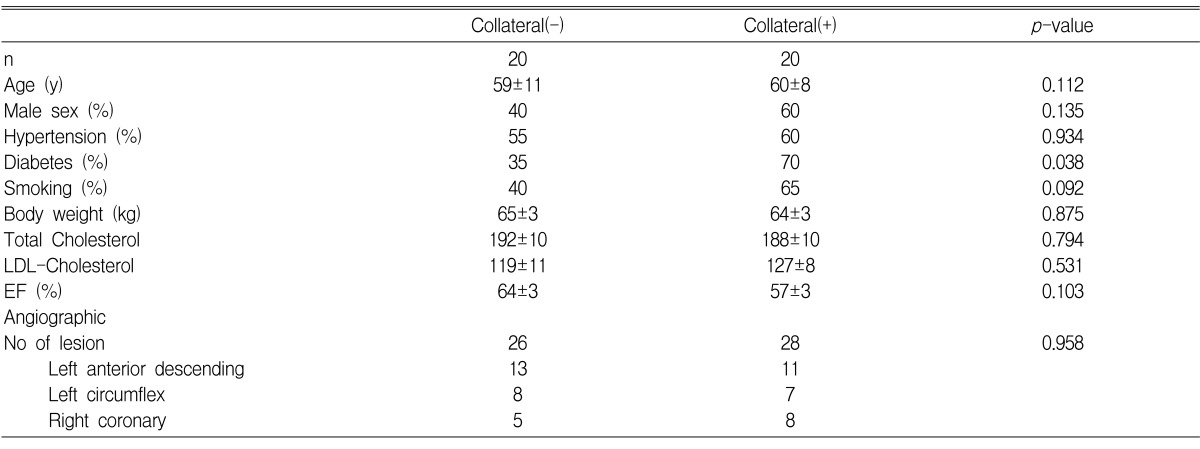
625 patients who had undergone coronary angiography at Hanyang University Hospital were recruited for our study. The exclusion criteria of this study were as follows: (i) stenosis of less than 90% on coronary angiography (541 patients); (ii) acute myocardial infarction (23 patients); and (iii) anemia, chronic inflammation and unknown data (21 patients). Finally, a total of 40 patients were enrolled in this study. The patients were assigned to the collateral circulation and non-collateral circulation groups. The collateral circulation group was 60% male and the non-collateral circulation group was 40%. No significant difference was noticed between the two groups (p=0.135). For risk factors like coronary artery diseases, including age, hypertension, smoking history, total cholesterol and low-density lipoprotein (LDL), there were no significant differences between the two groups. Diabetes mellitus was more prevalent in the collateral circulation group than in the non-collateral circulation group (p=0.038) (Table 2). There were no significant differences between the two groups for the cardiovascular drugs or heparin used prior to the coronary angiography. During an ecchocardiography, the ejection fraction showed no significant difference between the two groups (p=0.103).
Coronary artery collaterals were measured by the physician performing the catheterization, using visual analysis and using the conventional criteria. Regarding the coronary angiography, 40% of the 84 patients with a stenosis of more than 90% (34/84), had non-collateral circulation. Nineteen per cent of them (16/84) were Grade I and 40% of them (34/84) were Grade II. On the other hand, of the final 40 patients, 50% (20/40) were non-collateral circulation, 12% (5/40) were Grade I and 38% (15/40) were Grade II.
The expression of VEGF mRNA and HIF-1 (alpha) mRNA, depending on the collateral circulation development, are presented in Figure 2. For the subgroup analysis, the patient groups were classified into three subgroups on the basis of the modified TIMI system. There were no significant differences in the mean normoxic VEGF/18S and HIF-1 (alpha) mRNA value among the 3 collateral groups. The fold inductions of HIF-1 (alpha) mRNA under the hypoxic condition for 0+ collaterals, 1+ collaterals and 2+ collaterals were 1.20┬▒0.39, 1.28┬▒0.29 and 1.47┬▒0.63, respectively. This indicates there were no significant differences among the three groups (Figure 3). In contrast, the fold inductions of VEGF mRNA under hypoxic condition for 0+ collaterals, 1+ collaterals and 2+ collaterals were 1.65┬▒0.62, 3.77┬▒3.44 and 3.68┬▒3.43, respectively. This indicates that the collateral circulation group had a significantly higher fold inductions than the non-collateral circulation group (Figure 4).
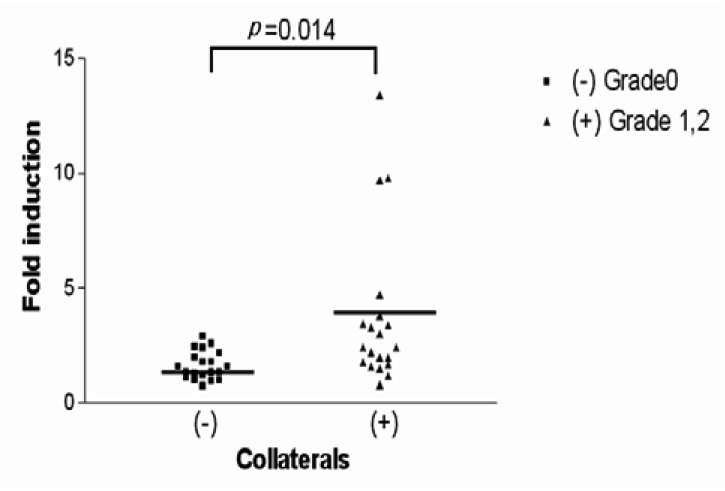
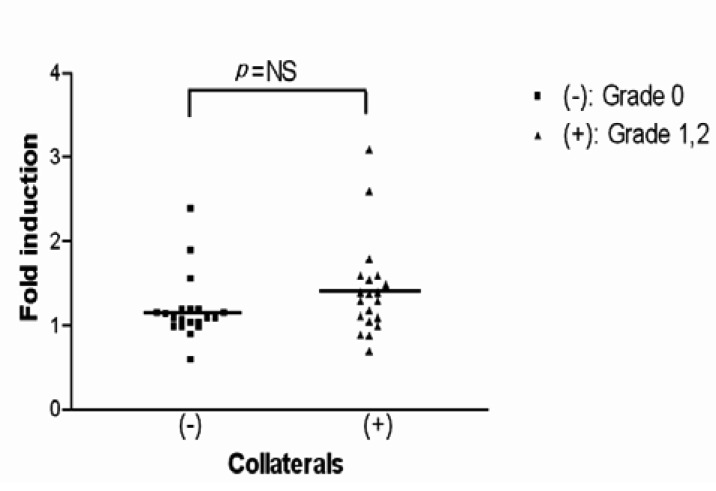
On the other hand, to minimize errors in evaluating the collateral circulation and to more accurately measure the HIF-1 (alpha) mRNA and VEGF mRNA levels (due to the collateral circulation formation), patients were allocated to the collateral circulation group and the non-collateral circulation group. Under the hypoxic condition, the fold inductions of HIF-1 (alpha) mRNA for the non-collaterals and collaterals were 1.20┬▒0.39 and 1.42┬▒0.57, respectively, indicating there was no significant difference between the two groups (p=0.165) (Figure 5). Conversely, under the hypoxic conditions, the fold inductions of VEGF mRNA for the non-collateral circulation group and for the collateral circulation group were 1.65┬▒0.62 and 3.70┬▒3.34, respectively, showing a significant difference between the two groups (p=0.014)(Figure 6, 7).
This study demonstrates that collateral circulation formation is associated with the expression of VEGF mRNA under hypoxic conditions, but not with the expression of HIF-1 mRNA.
When we assigned the patients to the three groups based on collateral circulation development, there was no significant difference in expression of VEGF mRNA among the three groups (under normal conditions). However, when under hypoxic conditions, the fold inductions of VEGF mRNA were significantly higher in the grade 2 group than in the Grade 0 group (p=0.037). To minimize errors in measuring collateral circulation development, patient groups were subclassified into collateral and non-collateral groups according to collateral circulation development. The fold inductions of the collateral circulation group were noted to be significantly higher than for the non-collateral circulation group (p=0.014).
The above result is significant since it was obtained after minimizing the error in evaluating the coronary angiographic findings. VEGF is assumed to have an important role in stimulating capillary angiogenesis in various organs. It also helps stimulate angiogenesis that minimizes cardiac injuries from a myocardial infarction. Local VEGF administration is known to stimulate endothelial proliferation and inhibit thrombogenesis10-13). From a collective analysis of previous studies, a hypoxic state stimulates local VEGF synthesis and involved in angiogenesis. Thus, collateral circulation formation takes place. The above hypothesis suggests that VEGF could have a therapeutic use, although there are conflicting opinions whether untoward vessels are formed. Also, it is uncertain whether VEGF is a sufficient factor for forming collateral circulation. Moreover, more human resources are required to study the therapeutic utility of VEGF.
This study supports the view that VEGF mRNA can be used therapeutically for non-responsive patients under hypoxic state by showing there is a correlation between the expressivity of VEGF mRNA and collateral circulation formation under a hypoxic state. There was no significant difference in the fold inductions of HIF-1 mRNA among the three groups. The fold inductions of HIF-1 mRNA were 1.20┬▒0.39 in the grade 0 group, 1.28┬▒0.29 in the grade 1 group and 1.47┬▒0.63 in the grade 2 group. In addition, subgroup analysis based on collateral circulation formation revealed that the fold inductions of HIF-1 mRNA were 1.20┬▒0.39 in the non-collateral circulation group and 1.42┬▒0.57 in the collateral circulation group. This indicates that the difference was not significant (p=0.165).
Therefore, this study indicated that collateral circulation formation was not associated with the expression of HIF-1 ╬▒ mRNA. Although the precise mechanism of VEGF expression remains unclear, it is assured that the elevated level of HIF-1 protein is essential for VEGF expression in ischemic myocardium. This theory is based on the grounds that HIF-1 regulates some part of the promoter region in VEGF1) and it stimulates VEGF expression in cardiac muscle cells cultured under a hypoxic state2). Lee et al.14) performed a histologic examination for cardiac muscle tissues on patients with myocardial infarction or acute cardiac ischemia. The results of Lee's study showed that HIF-1 mRNA and VEGF mRNA were sequentially expressed in cardiac muscle tissue from cardiac ischemia patients within 48 hours. Other tests indicate the same results occurred from the heart tissue of myocardial infarction patients within 24 hours before histologic examination. These were not expressed in normal cardiac muscle tissue or old infarcted cardiac muscle tissue. Based on the above results, Lee et al. stated that elevated levels of HIF-1 mRNA is an indicator for the early reaction of acute cardiac ischemia or myocardial infarction.
However, we confirmed that HIF-1 mRNA expression was not associated with hypoxia through in vitro monocyte cultures, which is consistent with the report by Wenger et al.15) Wenger et al. reported that VEGF mRNA and HIF-1 protein have increased binding activity but HIF-1 mRNA indicates no great change under hypoxic conditions in human Hep3B hepatoma cells. To explain these findings, Wengeret al. suggested that the DNA binding activity of HIF-1 may not follow the kinetics of HIF-1 ╬▒ mRNA.
On the other hand, Kawata et al.16) reported that the translocation of the PKC (protein kinase C) ╬Ą isoform stimulated VEGF mRNA expression and thereby stimulated angiogenesis. This leads to minimizing the infarcted lesion in a model of ischemic preconditioning for myocardial infarction, where the HIF-1 ╬▒ was not involved nor expressed in the infarcted cardiac muscle tissue. Rather, it acted independently of ischemic preconditioning or protein kinase C, which indicated that there was another mechanism of VEGF expression. This study indicated that the elevated levels of VEGF mRNA were detected with no increase in HIF-1 ╬▒ mRNA. This finding correlated significantly with the collateral circulation formation indicating that individual differences in the collateral circulation formation are associated with the expressivity of VEGF mRNA under a hypoxic state.
However, the expression of VEGF mRNA is assumed to be regulated not by the HIF-1 ╬▒ mRNA transcription level, but by other factors the including post-transcriptional modification process. In addition, this study showed there were no significant differences in factors such as age, gender, collateral circulation surgery history, hypercholesterolemia, hypertension and smoking between the collateral circulation group and the non-collateral circulation groups. These factors have been reported to be associated with collateral circulation formation in other studies17-20).
However, diabetes mellitus was more prevalent in the collateral circulation group than in the non-collateral circulation group. The difference was significant (p=0.038). For the subgroup analysis based on the presence of diabetes mellitus, the patients were divided into a diabetic group (n=21) and non-diabetic group (n=19). The prevalence of collateral circulation formation was significantly higher in the diabetic group (67%) compared to the non-diabetic group (32%) (p=0.041). But when we excluded diabetes, there was still a correlation between collateral formation and VEGF mRNA (p=0.049). The above result was similar to the report by Melidonis et al.21).
However, Waltenberger et al.22) reported that diabetic patients had insufficient collateral circulation formation and development because of impaired movement due to a derangement of the signal transduction system in the monocytes. The signal transduction system in the monocyte is known to be an important factor in collateral circulation development.
This study is somewhat inconsistent with Waltenberger et al.'s report. It is questionable whether such inconsistency reflects the standard errors from a small-sized sample of this study or the errors in the report by Waltenberger et al. Further investigation will be conducted for large-sized patient populations.
The limitations of this study were as follows. First, we measured the levels of HIF-1 ╬▒ mRNA and VEGF mRNA involved in the collateral circulation formation, but did not directly measure the protein of VEGF and HIF-1 ╬▒. Actually, we attempted to measure VEGF and HIF-1 ╬▒ proteins using antibodies but failed because of experimental errors. Second, this study recruited only a small-sized patient sample. Third, there may have been errors in the collateral circulation evaluation while determining the correlation between VEGF and HIF-1 ╬▒ mRNA.
We did not employ Laser Doppler Velocimetry or pressure wire, which are considered relatively accurate tools in the collateral circulation evaluation. However, any potential errors were minimized by assigning the patients to the collateral circulation and non-collateral circulation groups. Fourth, we cannot exclude the possibility of some changes occurred via in vitro hypoxia. It seems that a better model to examine the correlation with in vivo parameters would be to isolate the protein and mRNA from the recently/fresh isolated cells. Fifth, VEGF and VEGF-mRNA may be affected by serum heparin levels23, 24). However, it is assumed that any errors due to the difference in patients' heparin levels were adjusted for since there was no significant difference between the collateral circulation and non-collateral circulation groups.
In conclusion, the interindividual variability in the hypoxic induction of VEGF m-RNA in monocytes in patients is not controlled by the transcriptional levels of HIF-1 (alpha) with hypoxia. These findings suggest a mechanism such as the post-transcriptional modification of HIF-1(alpha) is involved in the hypoxic induction of VEGF.
References
1. Shima DT, Kuroki M, Deutsch U, Ng YS, Adamis AP, D'Amore PA. Themouse gene for vascular endothelial growth factor: genomic structure, definition of the transcriptional unit, and characterization of transcriptional and post-transcriptional regulatory sequences. J Biol Chem 1996;271:3877ŌĆō3883PMID : 8632007.


2. Levy AP, Levy NS, Loscalzo J, Calderone A, Takahashi N, Yeo KT, Koren G, Colucci WS, Goldberg MA. Regulation of vascular endothelial growth factor in cardiac myocytes. Circ Res 1995;76:758ŌĆō766PMID : 7728992.


3. Martin C, Yu AY, Jiang BH, Davis L, Kimberly D, Hohimer AR, Semenza GL. Cardiac hypertrophy in chronically anemicfetal sheep: increased vascularization is associated with increasedmyocardial expression of vascular endothelial growth factor andhypoxia-inducible factor 1. Am J Obstet Gynecol 1998;178:527ŌĆō534PMID : 9539521.


4. Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem 1994;269:23757ŌĆō23763PMID : 8089148.


5. Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasi. Curr Opin Genet Dev 1998;8:588ŌĆō594PMID : 9794818.


6. Hogenesch JB, Chan WK, Jackiw VH, Brown RC, Gu YZ, Pray-Grant M, Perdew GH, Bradfield CA. Characterization of a subset of the basichelix-loop-helix-PAS superfamily that interacts with components ofthe dioxin signaling pathway. J Biol Chem 1997;272:8581ŌĆō8593PMID : 9079689.


7. Yu AY, Frid MG, Shimoda LA, Wiener CM, Stenmark K, Semenza GL. Temporal, spatial, and oxygen-regulated expression of hypoxiainducible factor-1 in the lung. Am J Physiol 1998;275:L818ŌĆōL826PMID : 9755115.


8. Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest 1998;101:40ŌĆō50PMID : 9421464.



9. Schaper W, Buschmann I. Collateral circulation and diabetes. Circulation 1999;99:2224ŌĆō2226PMID : 10226084.


10. Asahara T, Bauters C, Pastore C, Kearney M, Rossow S, Bunting S, Ferrara N, Symes JF, Isner JM. Local delivery of vascular endothelialgrowth factor accelerates reendothelialization and attenuates intimal hyperplasia in balloon-injured rat carotid artery. Circulation 1995;91:2793ŌĆō2801PMID : 7758186.


11. Asahara T, Chen D, Tsurumi Y, Kearney M, Rossow S, Passeri J, Symes JF, Isner JM. Accelerated restitution of endothelial integrity andendothelial dependent function after phVEGF165 gene transfer. Circulation 1996;94:3291ŌĆō3302PMID : 8989143.


12. van Belle E, Tio FO, Couffinhal T, Maillard L, Passeri J, Isner JM. Stent endothelialization: time course, impact of local catheter delivery, feasibility of recombinant protein administration, and response tocytokine expedition. Circulation 1997;95:438ŌĆō448PMID : 9008462.


13. van Belle E, Tio FO, Chen D, Maillard L, Kearney M, Isner JM. Passivationof metallic stents after arterial gene transfer of phVEGF165 inhibits thrombus formation and intimal thickening. J Am Coll Cardiol 1997;29:1371ŌĆō1379PMID : 9137238.


14. Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, Thistlethwaite PA. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med 2000;342:626ŌĆō633PMID : 10699162.


15. Wenger RH, Kvietikova I, Rolfs A, Gassmann M, Marti HH. Hypoxia inducible factor-1(alpha) is regulated at the post m-RNA level. Kidney Int 1997;51:560ŌĆō563PMID : 9027739.


16. Kawata H, Yoshida K, Kawamoto A, Kurioka H, Takase E, Sasaki Y, Hatanaka K, Kobayashi M, Ueyama T, Hashimoto T, Dohi K. Ischemic preconditioning upregulates vascular endothelial growth factor mRNA expression and neovascularization via nuclear translocation of protein kinase C epsilon in the rat ischemic myocardium. Circ Res 2001;88:696ŌĆō704PMID : 11304492.


17. Ilia R, Carmel S, Gueron M. Patients with coronary collaterals and normalleft ventricular systolic function: clinical, hemodynamic, andangiographic characteristics. Angiology 1998;49:631ŌĆō635PMID : 9717893.


18. Kyriakides ZS, Kresmastinos DT, Michelakakis NA, Matsakas EP, Demovelis T, Toutouzas PK. Coronary collateral circulation in coronary artery disease and systemic hypertension. Am J Cardiol 1991;67:687ŌĆō690PMID : 1826068.


19. Yarom R, Zirkin H, Stammler G, Rose AG. Human coronary microvessels indiabetes and ischaemia: morphometric study of autopsy material. J Pathol 1992;166:265ŌĆō270PMID : 1517882.


20. Morimoto S, Hiasa Y, Hamai K, Wada T, Aihara T, Kataoka Y, Mori H. Influence factors on coronary collateral development. Kokyu To Junkan 1989;37:1103ŌĆō1107PMID : 2595120.

21. Melidonis A, Tournis S, Kouvaras G, Baltaretsou E, Hadanis S, Hajissavas I, Tsatsoulis A, Foussas S. Comparison of coronary collateral circulationin diabetic and nondiabetic patients suffering from coronary arterydisease. Clin Cardiol 1999;22:465ŌĆō471PMID : 10410290.



22. Waltenberger J. Impaired collateralvessel development in diabetes: potential cellular mechanisms and therapeutic implication. Cardiovasc Res 2001;49:554ŌĆō560PMID : 11166268.


Figure┬Ā1
Representative frames from patients with collaterals visualized by angiography. A grade of 0+ was given for no visible collaterals; 1+ for visible collateral but with no filling of the recipient epicardial vessels; and 2+ for filling (partial or complete) of the recipient epicardial vessel by the collaterals.
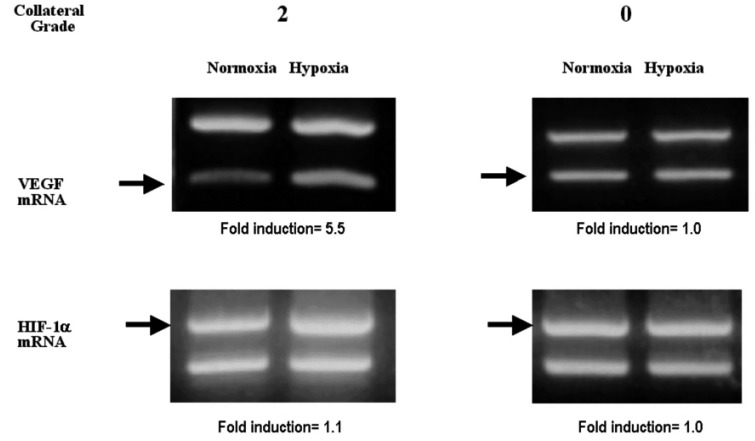
Figure┬Ā2
Representative RT-PCR assay demonstrating interindividual differences in the hypoxic induction of VEGF mRNA and HIF-1 mRNA. The fold induction of VEGF and HIF-1 mRNA by hypoxia was determined by dividing the hypoxic value by the normoxic value. For the representative patient with collateral grade 2 the fold induction of VEGF and HIF-1 mRNA was 5.5 and 1.1, respectively. But for the representative patient with collateral grade 0 the fold induction of VEGF and HIF-1 mRNA was 1.0.
Figure┬Ā3
The scattergram of the fold induction of VEGF mRNA by hypoxia in all patients included in this study separated by collateral score without adjustment for any of the covariates described. For the group with 0+ collaterals, the mean fold hypoxic induction of VEGF mRNA was 1.65┬▒0.62, for 1+ collaterals it was 3.77┬▒3.44 and for 2+ collaterals it was 3.68┬▒3.43. There was a statistically significant difference in the fold induction of VEGF mRNA between the group with 0+ collaterals and 2+ collaterals (p=0.037).

Figure┬Ā4
The scattergram of the fold induction of HIF-1 mRNA by hypoxia in all patients included in this study is separated by collateral score without adjustment for any of the covariates described. For the group with 0+ collaterals, the mean fold hypoxic induction of HIF-1 mRNA was 1.20┬▒0.39, for 1+ collaterals it was 1.28┬▒0.29 and for 2+ collaterals it was 1.47┬▒0.63. There was no statistically significant difference in the fold induction of HIF-1mRNA among the three groups (p=0.181).

Figure┬Ā5
The scattergram of the fold induction of VEGF mRNA by hypoxia in all patients included in this study is separated by collateral score without adjustment for any of the covariates described. For the group without collaterals, the mean fold hypoxic induction of VEGF mRNA was 1.65┬▒0.62, and for the group with collaterals it was 3.70┬▒3.34. There was a statistically significant difference in the fold induction of VEGF mRNA between the two groups (p=0.014).
Figure┬Ā6
The scattergram of the fold induction of HIF-1 mRNA by hypoxia in all patients included in this study is separated by collateral score without adjustment for any of the covariates described. For the group without collaterals, the mean fold hypoxic induction of HIF-1 mRNA was 1.20┬▒0.39 and for the group with collaterals it was 1.42┬▒0.57. There was no statistically significant difference in the fold induction of HIF-1 mRNA between the two groups (p=0.165).
Figure┬Ā7
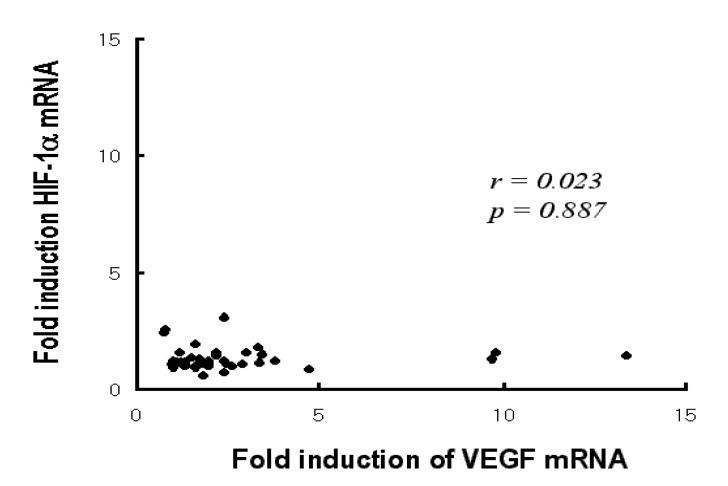
Correlation between the fold induction of VEGF mRNA and HIF-1 ╬▒ mRNA by hypoxia There was no statistically significant correlation between the fold induction of VEGF mRNA and HIF-1 ╬▒ mRNA (p=0.887).




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



