INTRODUCTION
The term ŌĆ£groove pancreatitisŌĆØ was first used in 1973 by Becker to describe a specific form of chronic pancreatitis that resulted in scarring, which extended into the anatomic space (ŌĆ£grooveŌĆØ) between the pancreatic head, duodenum, and common bile duct1). It is difficult to differentiate this disease from pancreatic cancer, preoperatively. Therefore, surgery was frequently performed in cases reported previously2). More recently, preoperative diagnosis of groove pancreatitis has been emphasized to avoid unnecessary radical surgery. Groove pancreatitis is not common, and only one case was recorded, but not published, in Korea. We report one case of segmental groove pancreatitis diagnosed by clinical and radiological features.
CASE REPORT
A 46-year old man was admitted to our hospital with severe postprandial abdominal pain in the right upper quadrant. He complained of right upper abdominal pain that lasted for six months and weight loss of 12 kg over the same period. During the last few weeks, he suffered from watery diarrhea without blood or mucus. He consumed about 40 g of ethanol per day for 20 years. Physical examination revealed tenderness in the right upper abdomen. The white blood cell count was 7,340/mm3 and the hematocrit was 39.1%. Serum pancreatic and hepatic enzymes were within normal range: amylase 62 IU/L (normal range 44ŌĆō128 IU/L), lipase 60 IU/L (normal range 7ŌĆō58 IU/L), total bilirubin 0.5 mg/dL (normal range 0.2ŌĆō1.2), alkaline phosphatase 69 IU/L (normal range 40ŌĆō122 IU/L), AST 12 IU/L (normal range 13ŌĆō36 IU/L), and ALT 20 IU/L (normal range 5ŌĆō44 IU/L). Serum glucose was 86 mg/dL. Tumor markers were also within normal range: carcinoembryonic antigen 1.46 ng/mL (normal range <5.0 IU/L) and carbohydrate antigen 19-9 3.17 U/mL (normal range <39 IU/L).
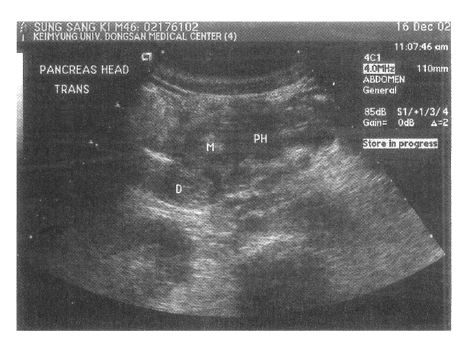
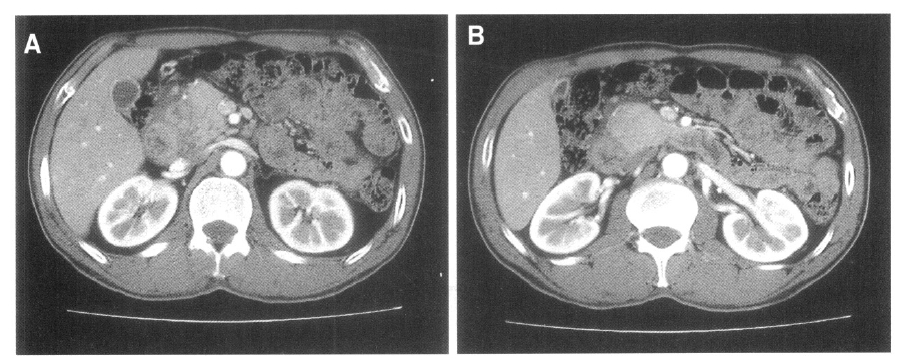
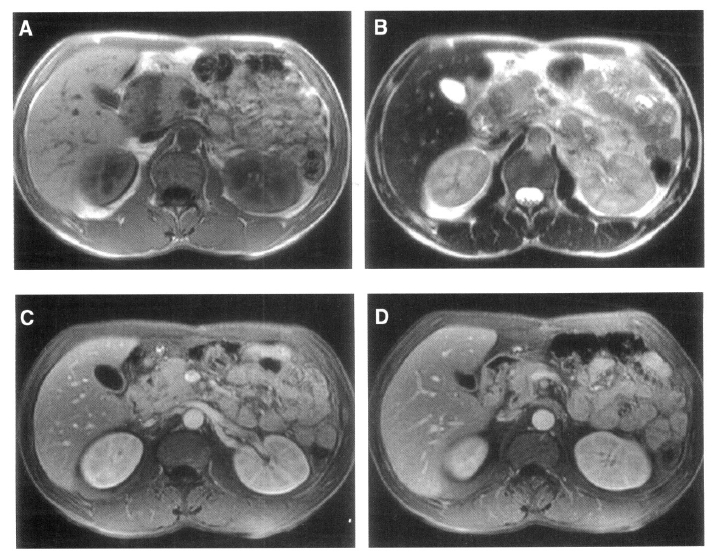
Abdominal ultrasonography showed a non-homogeneous mass measuring 32 mm in diameter between the pancreatic head, descending duodenum, and common bile duct, as well as swelling of the pancreatic head (Figure 1). Endoscopy revealed duodenal wall thickening adjacent to the major papilla, with narrowing of the duodenal lumen. Endoscopic biopsy showed only inflammation of the duodenal wall, without evidence of malignancy. Duodenal stenosis was not seen on duodenography. Computed tomography (CT) revealed swelling of the pancreatic head and a non-homogeneously enhanced, low-density area between the pancreatic head, descending duodenum, and common bile duct (Figure 2A). The head of the pancreas was enhanced on contrast CT scan and peripancreatic vessel encasement was not seen (Figure 2B). MR images revealed a mass between the pancreatic head, duodenum, and common bile duct that was hypointense on T1-weighted images (Figure 3A) and hypointense relative to the pancreatic head on T2-weighted images (Figure 3B). On T1-weighted images, we observed the medial wall thickening of the descending duodenum, several small cysts in the groove and thickened duodenal wall on enhanced images (Figure 3C, 3D). These radiological findings appeared consistent with the diagnosis of segmental groove pancreatitis. The patient has been under conservative treatment for 2 months and his severe abdominal pain has improved. Although he continues to have mild postprandial discomfort, he refuses evaluation and treatment for his disease.
DISCUSSION
Groove pancreatitis is a rare form of chronic pancreatitis affecting the groove between the pancreatic head, the duodenum, and common bile duct. There are two forms of groove pancreatitis: one is pure groove pancreatitis that only affects the groove, the other is segmental groove pancreatitis that affects the groove and the parenchyma of pancreas3,4).
Commonly reported symptoms include severe upper abdominal pain, recurrent postprandial vomiting, jaundice, diarrhea, and weight loss. Although the pathogenesis of groove pancreatitis remains unclear, it is suspected to be associated with previous history of disease in the biliary system, peptic ulcers, gastric resections, true duodenal wall cysts, pancreatic head cysts, pancreatic heterotopia in the duodenum, disturbed pancreatic juice flow in the Santorini duct in the absence of the minor papilla, and hypersecretion ancillary to alcohol abuse5,6). In one study3), 17 cases of groove pancreatitis were reviewed. The 17 patients had a median age of 51 years, and 16 of the 17 patients were alcoholics. Symptoms of abdominal pain were observed in 6, vomiting in 4 and jaundice in 2 of the patients. Preoperatively, almost all the patients were suspected of having pancreatic head cancer.
The diagnosis of groove pancreatitis is different according to each form. The pure form differentiates duodenal cancer, CBD cancer, and acute pancreatitis with phlegmon. For the segmental form, the most important differential diagnosis is pancreatic cancer. Characteristic findings on CT or MRI can suggest diagnosis of groove pancreatitis. While pancreatic cancer mass is seen as non-enhanced, in this disease, dynamic CT generally reveals an enhanced mass in the groove or the pancreatic head. Other findings suggestive of groove pancreatitis include cysts in the duodenal wall and/or the groove, and duodenal stenosis accompanied by wall thickening. Poor enhancement of scar lesions in groove pancreatitis may be due to delayed circulation caused by proliferation of fibrous tissues with attendant arterial constriction3,5,7). The most characteristic finding on MRI is a sheet-like mass between the head of the pancreas and the duodenum, associated with duodenal wall thickening. The mass is hypointense compared to pancreatic parenchyma on T1-weighted images, and iso- to slightly hyperintense on T2-weighted images. Dynamic imaging typically shows delayed enhancement8).
In our patient, the symptoms were postprandial right upper quadrant abdominal pain, watery diarrhea, and weight loss. He also had a long history of alcohol abuse. Abdominal CT revealed a non-homogeneously enhanced mass lesion at the groove and pancreatic head. We also observed cysts at the groove and duodenal wall. Abdominal CT revealed a mass with duodenal wall thickening, but endoscopic biopsy specimens obtained from the mucosa of the second portion of the duodenum only showed inflammation. Abdominal MRI revealed a sheet-like mass at the groove, associated with duodenal wall thickening and cysts. The mass was hypointense in T1-weigted images and had delayed enhancement. The peripancreatic vessel encasement was not shown. From these results, we were able to diagnose segmental groove pancreatitis preoperatively.
Although groove pancreatitis is not rare, only a few cases have been reported due to a lack of awareness. All clinicians should be familiar with its clinical features and keep them in mind for the differential diagnosis of pancreatic masses and duodenal stenosis. This may enable preoperative diagnosis and prevent unnecessary radical surgery.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





