 |
 |
| Korean J Intern Med > Volume 8(2); 1993 > Article |
|
Abstract
To elucidate the nature of altered cellular immunity seen in patients with chronic renal failure, the values of interleukin-2 (IL-2), a kind of lymphokine, and T-cell colony forming units were measured in controls (N=10), predialysis uremic patients (N=14), patients undergoing chronic hemodialysis (HD, N=11) and patients on continuous ambulatory peritoneal dialysis (CAPD, N=9). Dialytic patients were selected as relatively stable cases receiving dialysis for more than 3 months. The duration of dialysis was 25.5±5.5 months in HD and 14.7±3.0 months in CAPD groups. The mean age was 30.3 years in the control, 36.1 years in the predialysis, 32.9 years in the HD and 41.1 years in the CAPD groups; all 4 groups showed male predominance. The serum creatinine concentration of each group was 1.2±0.1 mg/dl in the control, 14.1±0.9 mg/dl in predialysis, 13.5±1.3 mg/dl in HD and 14.7±0.9 mg/dl in CAPD groups. The level of IL-2 in the predialysis group was markedly lower compared to the control, HD and CAPD groups (as 3.1±0.8 unit vs. 8.8±2.2 unit, 11.8±3.0 unit and 14.9±3.4 unit, respectively, p<0.05); the difference between the control and dialytic groups was not statistically significant. The value of the T-cell colony forming unit in the predialysis group was far lower than the other 3 groups (as 427±69 colony/petri dish vs. 998±263 colony/petri dish in control, 1114±273 colony/petri dish in HD and 1369±372 colony/petri dish in CAPD groups, respectively, p<0.05), whereas there was no significant difference between the control and either HD or CAPD groups.
In conclusion, the lymphokine productivity, as reflected by the level of IL-2 and the T-cell colony forming unit, seemed to be highly suppressed in predialysis uremic patients compared to control and dialytic patients. This result suggests that dialytic treatment tends to improve IL-2 productivity and T-cell colony forming unit, but further evaluations are needed to confirm these finding.
Patients with chronic renal failure manifest significant functional alterations of the immune systems1,2). The dominant immunologic defect resides in the cell-mediated immunity which primarily involves T lymphocytes3–5). Many studies show cutaneous anergy6), prolonged allograft survival7,8), a decreased antibody response to thymus dependent antigens like vaccination with HB virus9,10), influenza virus11), an increased incidence of infection such as mycobacteriosis12,13) and altered tumor surveillance system14,15). This suppressed cellular immunity can be explained by decreased absolute T lymphocyte numbers16), increase in suppressor T cell activity5) and inhibitory effect of uremic serum on lymphocyte blastogenesis17). The mechanism underlying this cellular immune defect in uremia is not well understood yet. The possibility has been raised to explain the immunosuppression associated with chronic renal failure, including an intrinsic defect of uremic effector T lymphocytes18) and/or the presence of an abnormal immunoregulation mediated by regulatory T lymphocytes (such as, helper and suppressor T cells), monocytes or soluble serum factors19–22).
Recent studies have indicated that clonal expansion of T lymphocytes, after antigen or mitogen triggering, requires the presence of a soluble mediator or interleukin23). In addition, such a factor also appears to be necessary for differentiation of precytotoxic T lymphocytes into cytotoxic T lymphocyte effector cell24). Several lines of evidence support the notion that a single T cell-derived glycoprotein, termed interleukin-2, possesses both activities in humans as well as experimental animals25,26). Moreover, several techniques have been described for growth of human T lymphocytes colonies in soft agar27–29). Goube de Laforest et al30) have emphasized the role that cell interactions play in T-cell colony formation and have suggested that the three cell population might cooperate in the primary culture of T-cell colonies. As part of the screening of immune function, T-cell colony formation may be an invaluable measure of cell-mediated immunity.
The purpose of this study is to elucidate the nature of altered immune functions seen in uremic patients by studying IL-2 production and T cell colony forming unit in patients with chronic renal failure.
Ten healthy normal controls and thirty four patients with end stage renal failure were selected from those who gave informed consent to this study at the dialysis centers of Yongdong Severance Hospital and Severance Hospital, Yonsei University Medical Center. Dialytic patients were selected as relatively stable cases receiving dialysis for more than 3 months. They ranged in age from 20 to 55 years. Patients with end stage renal failure were subdivided by three groups; i.e., predialysis group (14 patients), hemodialysis group (11 patients) and CAPD group (9 patients) (Table 1). Table 2 summarizes BUN, creatinine and duration of dialysis recorded at the time of the blood collected.
Lymphocytes were separated from whole heparinized blood on Ficoll-Hypaque density gradient. The cell layer was washed three times in Hanks solution and suspended in mcCoys 5A medium. The final cell counts of suspension were adjusted to 5×103 cells/ul.
Separated lymphocytes were floated at 2×106 cells/ml in final culture medium RPMI 1640 with 2% human AB serum, penicillin (100 unit/ml), streptomycin (100 unit/ml) and L-glutamine (2 mM) in the presence of 10 ug/ml phytohemagglutinin. Cell suspensions were placed by 1 ml per each well at 24 well tissue culture clusters and after 24 hours in culture at 5% CO2 at 37°C humidified atmosphere, cells were pelleted by centrifugation. The supernatants were stored at −70°C for future IL-2 assay.
IL-2 activity was measured by proliferation of IL-2 dependent murine cytotoxic T cell (CTLL-2) line. Serial dilution of supernatants in RPMI 1640, supplemented with 10% human AB serum and 50 mM 2-mercaptoethanol, were incubated 20 hours with 10,000 CTLL-2 cells in a total volume of 100 ul in 96 well culture plates at 5% CO2 at 37°C humidified atmosphere. Cultures were pulsed after 20 hours with 1 uCi H3-thymidine per well and harvested 4 hours later. H3-thymidine (New England Nuclear, Boston, MA, USA) incorporation was measured in a Packard scintillation counter (Packard Co., Downer’s Grove, IL, USA). Each value represents the mean of triplicates. IL-2 activity was calculated using standard curve obtained by 1 unit per 50% of maximum H3-thymidine uptake of CTLL-2 culture.
The culture method used was the agar gel system for culturing T cells using plastic petri dishes of 15×60 mm (Falcon). The agar layer in each petri dish consisted of 4 ml of medium composed of RPMI 1640 with 0.7% agarose solution and 30 minutes before using, petri dish was kept in 5% CO2 at 37°C humidified atmosphere. 200 ul (1.5×106 cells) of lymphocytes suspension was mixed with 15 ul PHA (225 ug/ml), 15 ul HEPES (1 M) and 300 ul human AB serum. This mixture, with 1 ml 0.5% agarose solution, was permitted to gel at 24°C for 15 minutes. The dishes were incubated at 37°C in a water saturated atmosphere with 5% CO2. After 7 days incubation, the development of clones and their morphology were observed under an inverted microscope using a magnification of 100 X. A cell mass exceeding 15 cells was identified as a T cell colony. The total number of colonies per petri dish was calculated as an index of colony forming capacity. For each experiment, three replicate plates were scored and made a mean.
The IL-2 production and T cell colony forming unit of patients with chronic renal failure and the control are summarized in Table 3. The level of IL-2 of the normal control group was 8.8±2.2 (Mean±SE), and the level of IL-2 of the predialysis uremic, hemodialysis and CAPD groups were 3.1±0.8, 11.8±3.0 and 14.9±3.4 respectively. The level of IL-2 in the predialysis uremic group was significantly lower compared to the control, hemodialysis and CAPD groups (p<0.05). The difference of the level of IL-2 between the control and dialytic groups was not statistically significant (Fig. 1). The value of T cell colony forming unit in the control, predialysis uremic, hemodialysis and CAPD groups were 998±263, 427±69, 1114±273 and 1369±372, respectively. The value of T cell colony forming unit in the predialysis group was far lower than the other 3 groups (p<0.05), whereas there was no significant difference between the control and either hemodialysis or CAPD group (p>0.1).
Many studies on the immunologic function of patients with chronic renal failure have showed the findings of lymphopenia16,31), reduced cutaneous hypersensitivity to antigens6,32,33), frequent infections12,34,35), prolonged skin allograft survival7,8,36), a decreased antibody response to thyrnus derived antigen, like vaccination with HB virus8–10), and increased incidence of malignancies14,15). The dominant defects in the immune system of these patients reside in the cell-mediated immunity which primarily involves T lymphocytes. Recently, using monoclonal antibodies, the T-cell subsets in the peripheral blood of uremic patients have already been examined19,20,37) and the mitogenic response of uremic lymphocytes have also been done6,38–42). The average percent of suppressor cells in a uremic patient is normal according to some authors19,20) and decreased according to others37,43). Earlier studies of the in-vitro response of uremic lymphocytes to mitogens and allogeneic antigens in human patients have reported controversial results: the mitogenic responses have been variously reported as reduced6,38–40,44), normal45,46) or even increased42) However, few studies were performed to measure the productivity of interleukin-2, which is a very important lymphokine produced mainly by T helper cell and T cell colony forming unit in patients with chronic renal failure.
We targeted our attention on the ability of lymphocytes to produce interleukin-2 and T cell colony formation, which is a sensitive measure of cell-mediated immunity29). Interleukin-2 is essential for appropriate cell cooperation in delayed type hypersensitivity reactions known to be severely impaired in patients with chronic renal failure. Lamperi and Carozzi (1985)47) demonstrated that the TCGF (T cell growth factor) activity was, in uremic patients in predialysis phase or on HD, reduced values in comparison to the levels observed in normal control. Kurz et al (1986)18) also showed that, although without statistical significance, there was a tendency by uremic peripheral blood lymphocytes to produce less IL-2 as compared to healthy controls. Beaurain et al (1989)48) showed again that IL-2 activity was significantly decreased in predialysis and hemodialysis uremic patients compared to healthy normal controls. But Kimmel et al (1989)49) reported contradictory results that all of the uremic groups (including dialytic groups) showed increased IL-2 production capacity compared to normal controls. Our results, obtained in this study, show abnormally suppressed interleukin-2 productivity in the predialysis uremic group. Dialytic groups revealed increased interleukin-2 productivity, which was even higher compared to control but statistically non-significant. Our present data are in good agreement with Lamperi and Carozzi (1985)47), Kurz et al (1986)18) and Beaurain et al (1989)48).
In 1976, Fibach et al27) showed that in-vitro colony formation with normal human lymphocytes should be of value for studies on antigenicity, the immune response and the results of virus infection and lymphocytes diseases. Colony formation with normal lymphocytes might be induced in semisolid medium by providing the appropriate antigenic stimulus. Studies with different concentrations of PHA have shown that the number of colonies formed was related to lectin concentration. T-cell colony forming cells are thought to be non-cycling small lymphocytes of a density of 1.069–1.077 g/cm3,28). In 1989, Wakabayashi et al50) reported that colony formation was markedly reduced in chronic renal failure patients in comparison with normal control, with about half of the patient-group showing no colony growth. All cases showed a significant increase in colony numbers with in-vitro plasmapheresis and with the addition of exogenous interleukin-2. These results suggest that T-cell precursors exist in near normal numbers in chronic renal failure patients and that there are humoral inhibitors in the uremic plasma. Our results also showed that predialysis uremic patients revealed far lower T-cell colony forming units compared to the other 3 groups and there were no significant differences between the control and either HD or CAPD groups. These results suggest that IL-2 productivity and T-cell colony forming unit of the patients with predialysis uremic group seemed to be highly supressed compared to the control and dialytic groups. And also, dialytic treatment may be improve IL-2 productivity and the T-cell colony forming unit of the patients with predialysis uremic group.
In conclusion, the IL-2 productivity and the T-cell colony forming unit seemed to be highly suppressed in predialysis uremic patients compared to control and dialytic patients. Dialytic treatment tends to improve IL-2 productivity and T-cell colony forming unit, but further evaluations are needed to confirm these finding.
Fig. 1.
Interleukin 2 productivity of the patients group and the control.
*: p<0.05, compared with values in the control, hemodialysis and CAPD group.
HD: hemodialysis, CAPD: continuous ambulatory peritoneal dialysis.
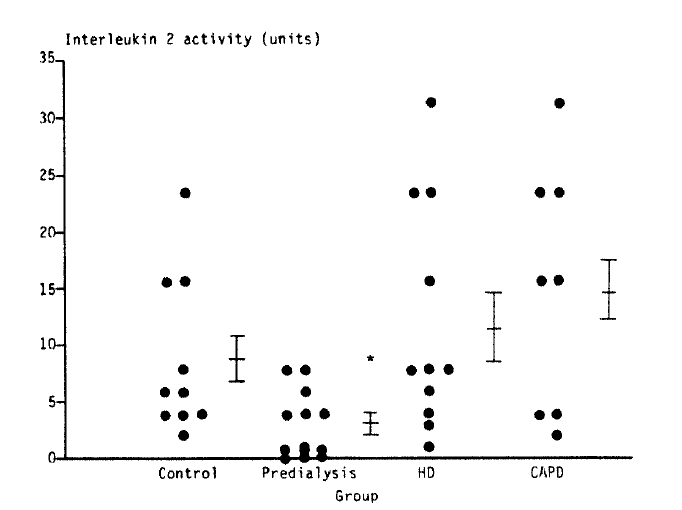
Fig. 2.
T-cell colony forming unit of the patients group and the control.
TCFU: T-cell colony forming unit.
*: p<0.05, compared with values in the control, hemodialysis and CAPD group.
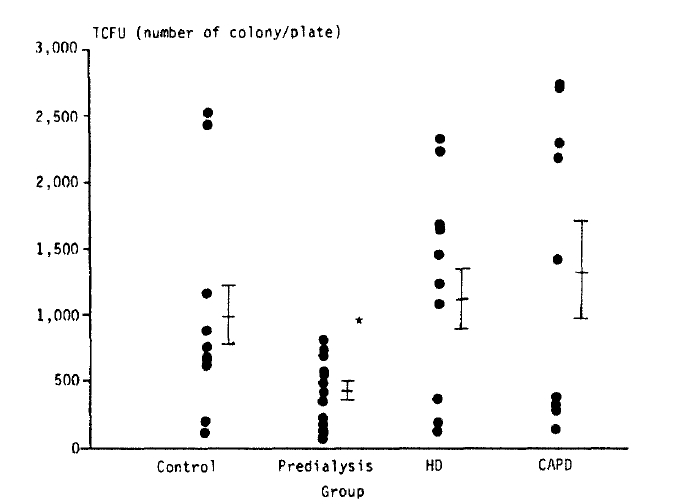
Table 1.
Age and Sex Distribution of the Subjects
| Group | No. of Subjects (Male, Female) | Age | |
|---|---|---|---|
| Mean | Range | ||
| Control | 10 (9, 1) | 30.3±2.6 | 20–40 |
| Predialysis | 14 (8, 6) | 36.1±2.7 | 21–53 |
| Hemodialysis | 11 (6, 5) | 32.9±2.7 | 25–55 |
| CAPD | 9 (7, 2) | 41.1±2.8 | 30–54 |
Table 2.
BUN, Creatinine, and Duration of Dialysis of the Subjects
| Group | BUN (mg/dl) | Creatinine (mg/dl) | Duration of Dialysis (month) |
|---|---|---|---|
| Control | 14.6± 0.7 | 1.2±0.1 | |
| Predialysis | 103.6±10.5 | 14.1±0.9 | |
| Hemodialysis | 85.5± 8.5 | 13.5±1.3 | 25.5±5.5 |
| CAPD | 54.9± 3.7 | 14.9±0.9 | 14.7±3.0 |
REFERENCES
1. Boulton-Jones JM, Vick R, Cameron JS, Black PJ. Immune response in uremia. Clin Nephrol 1:30. 1973.
2. Goldblum SE, Reed PW. Host defenses and immunologic alterations associated with chronic hemodialysis. Ann Intern Med 93:597. 1980.


3. Morrison AB. Immunological disturbances in uremia. In: Massry SG, Glassok RJ, eds. Textbook of nephrology. 7–147Baltimore: Williams & Wilkins, 1983.
4. Osaki K, Otuska H, Uomizu K, Harada R, Otsuji Y, Hashimoto S. Monocyte-mediated suppression of mitogen responses of lymphocytes in uremic patients. Nephron 34:87. 1983.


5. Smith MD, Hardy G, Williams JD, Coles GA. Suppressor cell numbers and activity in non-transfused renal dialysis patients. Clin Nephrol 3:130. 1983.
6. Selroos O, Pasternack A, Virolainen M. Skin test sensitivity and antigen-induced lymphocyte transformation in uremia. Clin Exp Immunol 14:365. 1973.


7. Dammin GJ, Couch NP, Murray JE. Prolonged survival of skin homografts in uremic patients. Ann NY Acad Sci 64:967. 1967.

8. Morrison AB, Manness K, Tawes R. Skin homograft survival in chronic renal insufficiency. Arch Pathol 75:139. 1963.
9. Kohler H, Arnold W, Renschin G, Dormeyer HH, Meyerzum Buschenfelde KH. Active hepatitis B vaccination of dialysis patients and medical staff. Kidney Int 25:124. 1984.


10. Stevens CE, Alter MD, Taylor PE, Zang EA, Harley EJ, Szutiuness W. Hepatitis-B vaccine in patients receiving hemodialysis. Immunogenicity and efficacy. N Engl J Med 311:496. 1984.


11. Cappel R, Van Beers D, Liesnard C, Dratwa M. Impaired humoral and cell-mediated immune responses in dialyzed patients after influenza vaccination. Nephron 33:21. 1983.


12. Andrew OT, Schoenfeld PY, Hopewell PC, Humphreys MH. Tuberculosis in patients with end-stage renal disease. Am J Med 68:59. 1980.


13. Lundin AP, Adler AJ, Berlyne GM, Friedman EA. Tuberculosis in patients undergoing maintenance hemodialysis. Am J Med 67:597. 1979.


15. Linder A, Farewell VT, Sherrard DJ. High incidence of neoplasia in uremic patients receiving long term dialysis. Nephron 27:292. 1981.


16. Wilson WEC, Kirkpatrick CH, Talmage DW. Suppression of immunologic responsiveness in uremia. Ann Intern Med 62:1. 1965.

17. Newberry WM, Sanford JP. Defective cellular immunity in renal failure. Depression of reactivity of lymphocytes to phytohemagglutinine by renal failure serum. J Clin Invest 50:1262. 1971.



18. Kurz P, Kohler H, Meuer SC, Hutteroth TH, Meyer Zum, Buschenfelde KH. Impaired cellular immune responses in chronic renal failure: Evidence for a T-cell defect. Kidney Int 29:1209. 1986.


19. Lortan JE, Kiepiela P, Coovadia HM, Seedat YK. Suppressor cells assayed by numerical and functional tests in chronic renal failure. Kidney Int 22:192. 1982.


20. Raskova J, Ghobrial I, Shea SM, Eisinger RP, Raska K Jr. Suppressor cells in End stage renal disease. Functional assays and monoclonal antibody analysis. Am J Med 76:847. 1984.


21. Raskova J, Morrison AB. A decrease in cell-mediated immunity in uremia associated with an increase in activity of suppressor cells. Am J Pathol 84:1. 1976.


22. Raskova J, Raska K Jr. Humoral inhibitors of the immune response in uremia. V. Induction of suppressor cells in vitro by uremic serum. Am J Pathol 111:149. 1983.


23. Watson J, Mochizuki D. Interleukin 2: A class of T-cell growth factors. Immunol Rev 51:257. 1980.


24. Wagner H, Hardt C, Heeg K, Rollinghoff M, Pfizenmaier K. T-cell derived helper factor allows in vivo induction of cytotoxic T-cells in nu/nu mice. Nature 284:278. 1980.


25. Gillis S, Gillis AE, Henney CS. Monoclonal antibody directed against interleukin 2. I. Inhibition of T lymphocyte mitogenesis and the in-vitro differentiation of alloreactive cytotoxic T-cells. J Exp Med 154:983. 1981.



26. Kern DE, Gillis S, Okada M, Henney CS. The role of interleukin-2 (IL-2) in the differentiation of cytotoxic T-cells: The effect of monoclonal anti-IL-2 antibody and absorption with IL-2 dependent T-cell lines. J Immunol 127:1323. 1981.

27. Fibach E, Gerassi E, Sachs L. Induction of colony formation in-vitro by human lymphocytes. Nature 259:127. 1976.


28. Claesson MH, Rodger MB, Johnson GR, Whittingham S, Metcalf D. Colony formation by human T lymphocytes in agar medium. Clin Exp Immunol 28:526. 1977.


29. Shen J, Wilson F, Shifrine M, Gershwin ME. Select growth of human T lymphocytes in single phase semisolid culture. J Immunol 119:1299. 1977.



30. Goube de Laforest P, Thomas P, Pelletier D, Tanzer J. Studies on cell interactions using a micromethod for growing T-lymphocytes colonies in agar culture. J Immunol 38:561. 1979.
31. Jensson O. Observations on the leukocyte blood picture in acute uremia. Br J Haematol 4:442. 1958.

32. Huber H, Pastner D, Dittrich P, Braunsteiner H. In-vitro reactivity of human lymphocytes in uremia-a comparison with the impairment of delayed hypersensitivity. Clin Exp Immunol 5:75. 1969.


33. Casciani CU, Desimon C, Bonni S, Galluci M, Mottencci G, Mieli D, Masala C. Immunological aspect of chronic uremia. Kidney Int (Suppl) 8:s49. 1978.
35. Rutsky EA, Rostand SG. Mycobacteriosis in patients with chronic renal failure. Arch Intern Med 140:57. 1980.


36. Smiddy FG, Burwell RG, Parsons FM. Influence of uremia on the survival of skin homograft. Nature 190:732. 1961.

37. Bender BS, Curtis JL, Nagel JE, Chrest FJ, Kraus ES, Brieffel GR, Adler WH. Analysis of the immune status of hemodialyzed adults: association with prior transfusions. Kidney Int 26:436. 1984.


38. Kauffman CA, Manzler AD, Phair JP. Cell-mediated immunity in patients on long term hemodialysis. Clin Exp Immunol 22:54. 1975.


39. Miller TE, Stewart E. Host immune status in uremia I. Cell-mediated immune mechanisms. Clin Exp Immunol 41:115. 1980.


40. Kunori T, Fehrman I, Ringden O, Moller E. In-vitro characterization of immunological responsiveness of uremic patients. Nephron 26:234. 1980.


41. Kasakura S, Lowenstein L. The effect of uremic blood on mixed leukocyte reactions and on cultures of leukocytes with phytohemagglutinin. Transplantation 5:283. 1967.

42. Daniels JC, Sakai H, Rerumiers AR Jr, Sarles HE, Fish JC, Cobb EK, Levin WC, Ritzman SE. In-vitro reactivity of human lymphocyte in chronic uremia: analysis and interpretation. Clin Exp Immunol 8:213. 1971.


43. Collart F, Tielemaus C, Schandene L, Dupout E, Wybrau J, Dratwa M. CAPD and celluar immunity: no different than that in hemodialysis patients. Perit Dial Bull 3:163. 1983.

45. Sengar DPS, Hyslop DB, Rashid A, Harris JE. T-rosettes in hemodialysis patients and renal allograft recipients. Cell Immunol 20:92. 1975.


46. Byron PR, Mallick NP, Taylor G. Immune potential in human uremia. I. Relationship of glomerular filtration rate to depression of immune potential. J Clin Path 29:765. 1976.



47. Lamperi S, Carozzi S. T lymphocytes, monocytes and erythropoiesis disorders in chronic renal failure. Nephron 39:211. 1985.


48. Beaurain G, Naret C, Marcon L, Grateau G, Drueke T, Urena P, Nelson DL, Bach JF, Chatenoud L. In-vitro T-cell preactivation in chronic uremic hemodialyzed and non-hemodialyzed patients. Kidney Int 36:636. 1989.


49. Kimmel PL, Phillips T, Bosch JP. Lymphokines in renal disease (RD): Uremia (U), hemodialysis (HD) and CAPD. Kidney Int 35:195. 1989.
50. Wakabayashi Y, Sugimoto M, Horie S, Abe S, Hirose S, Okuda T. Studies on T-cell Colony formation in chronic renal failure (CRF) patients. Clin Nephrol 12:270. 1989.
-
METRICS

- Related articles
-
The Studies on the Gastrin Levels in the Patients with Renal Failure1986 January;1(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


