The first description of a group of patients with finger ischemia presumably caused by digital artery vasospasm was published by Raynaud1) in 1862. In 1932, Allen and Brown2) emphasized the wide spectrum of the disorders and attempted to distinguish the Raynaud’s phenomenon from the Raynaud’s disease. They defined Raynaud’s disease as a vasospastic episode precipitated by cold or emotions, usually being bilateral, associated with little or no gangrene, and having no underlying medical disease for more than 2 years. Raynaud’s phenomenon, on the other hand, was a marker for a variety of underlying medical conditions, frequently asymmetric, involving fewer digits, and having an increased risk of developing gangrene.
A wide variety of medical conditions have been associated with Raynaud’s phenomenon. The general categories include connective tissue disease, hematologic abnormalities, arterial diseases, neurologic disorders, occupational causes, drugs and toxins. Among them, the connective tissue diseases are most frequently linked with Raynaud’s phenomenon.
Raynaud’s phenomenon is frequently the first symptom of several connective tissue diseases. Seventy to 80 percent of scleroderma patients present with Raynaud’s phenomenon3). Several factors controlling the finger blood flow include intrinsic vascular tone4), adrenergic activity5,6), blood viscosity7–10) and body fluid components etc11,12).
A variety of methods to establish objective diagnostic criteria include immunological tests, nerve conduction studies, thermocouple (Porter et al)13), thermography (Chucker et al)14), calorimetry (Mendlowitz et al)15), plethysmography (Sumner and Strandness et al)16), ultrasound, laser doppler flowmetry (Wigley et al)17), magnification and cryodynamic hand arteriography (Porter & Rosch et al)18), and measuring blood viscosity (Pirofsky et al)7).
Coffman and Cohen used regional isotope clearance to measure capillary flow by monitoring the disappearance rate of Na131I in saline which was injected into the pad of a finger tip19). Lim and his colleagues reported the radionuclide angiographic method using technetium 99m-red blood cell in 198720).
We measured cumulative radioactivity which was the integrated radioactivity of each hand for 310 seconds after intravenous bolus injection of 99mTc-MDP. Cumulative blood flow ratio of the cold exposed hand to room air exposed hand, measured by Tc-99m MDP, was considered as an efficient, economical and new method to evaluate the finger blood flow.
METHODS AND MATERIALS
Forty two patients from the the hospitalized population at the National Medical Center in Seoul were included in the study. At the time of entry into the study, all patients were classified into 3 groups. 10 patients who were dignosed to have associated connective tissue diseases were classified as having Raynaud’s phenomenon (group 1). They had Raynaud’s history and symptoms, and showed typical color change in our cold water challenge. 12 patients had connective diseases without Raynaud’s phenomenon (group 2) according to the criteria of Allen and Brown2). Normal control group consisted of 20 persons who had no evidence of Raynaud’s phenomenon or connective tissue disease (group 3) by history or serologic study.
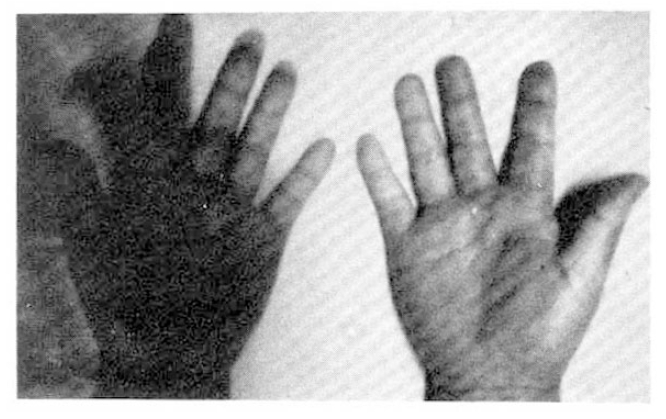
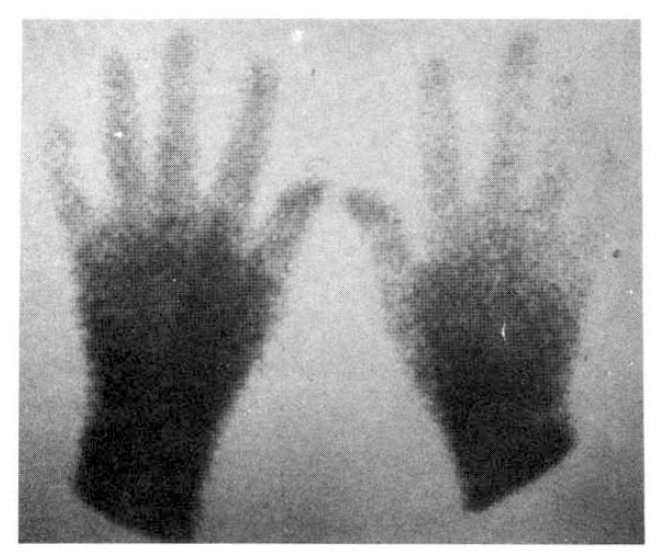
On the day of the radionuclide imaging, all patients rested in sitting position for 20 minutes in a room whose temperature was maintained at 22°C. After that, they immersed one of their hands into the ice water (4°C) to the wrist for 30 seconds, and sat in that room for another 15 minutes with their hands at the heart level. The patients were instructed not to wipe or shake off the water from their hands. Then, they all received 20 μCi of Tc-99m MDP by IV bolus at the ante-cubital fossa. We got scintigraphic image of both hands with the region of interest (ROI), including the second, third, fourth and fifth fingers distal to MCP joint in each hand (Fig. 1). Computer recording of activity at each ROI every 2 seconds for 310 seconds were started just after IV bolus injection. We also observed if the patients showed typical skin color change of hands and symptoms (Fig. 2). Repeated imaging was done in 10 patients with Raynaud’s phenomenon after the administration of calcium antagonist (nifedipine 30–60 mg/day), vasodilator (captopril 50 mg or doxazosin 1–6 mg/day), and antiplatelet druge (aspirin 300 mg/day) for about 2 weeks.
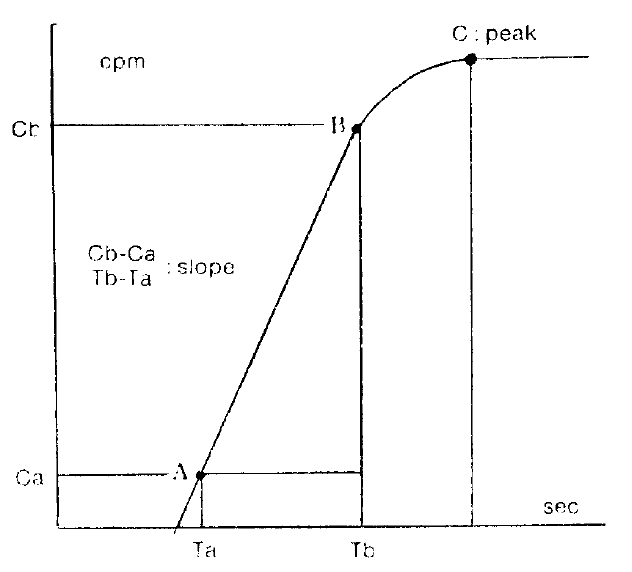
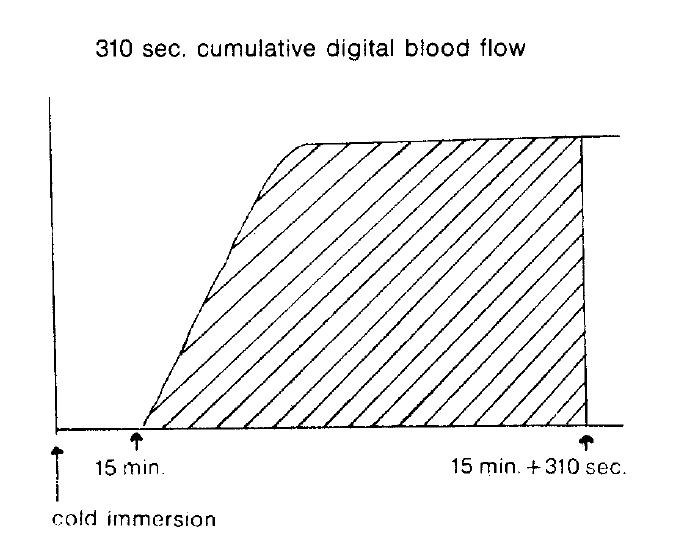
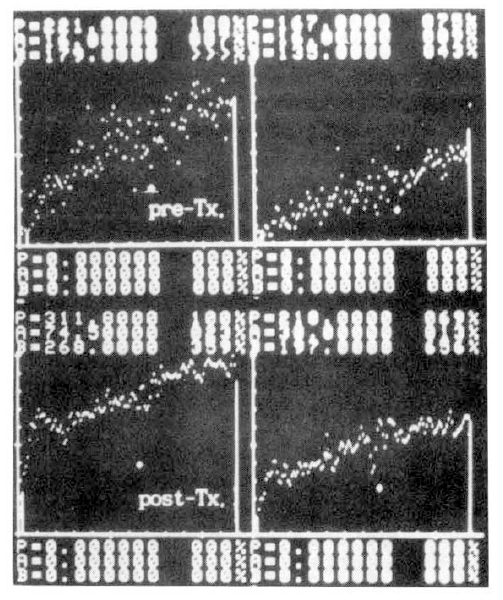
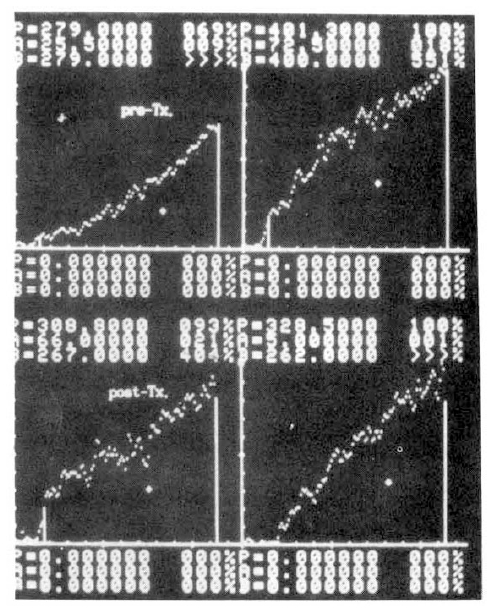
Several parameters were used in this study. The first was the ratio of activity in cold exposed hand to room air exposed hand, 15 minutes and 20 minutes after cold provocation. The second was uptake increase in the same hand, room air or cold water exposed hands, respectively, from the time of 50 counts per minute (CPM) in cold exposed hand to 310 seconds. The third was initial slope ratio which is the ratio of initial slope of cold exposed hand to room air exposed hand. The slope of activity curve from the beginning of uptake to the initial peak was compared between the two hands (Fig. 3). The fourth was 310 second cumulative digital blood flow ratio of cold water exposed hand to room air exposed hand. The areas below the time activity curve of the region of interest of each hands for 310 seconds from IV bolus injection of 99mTc-MDP was considered as cumulative blood flow (Fig. 4). One way ANOVA test was used for statistical evaluation purposes. Results were given as mean±SD.
RESULTS
The study population’s clinical and laboratory findings are given in Table 1. The comparison of several parameters of both hands was done (Table 2). The ratio of activity in cold exposed hand to room air exposed hand were 0.329±0.315 (mean±SD) in group 1, 0.534±0.242 in group 2, 0.549±0.053 in group 3, 15 minutes after their cold exposure, respectively. There was no statistical significance among groups. As in the initial slope ratio, the ratio of activity of patients with Raynaud’s pheomenon (0.427±0.156) (group 1) was lower than that of normal control group (0.739±0.185). The differences were p<0.001. Otherwise, the initial slope of group 2 was 0.829±0.161. No significant difference was noted between group 2 and 3. In 310 second cumulative digital blood flow ratio, the ratio of Raynaud’s was 0.456±0.104. The ratio of normal control group was 0.817±0.106. There was a high significance (p<0.001). 310 cumulative digital blood flow was an integrated value of count graph for 310 seconds.
Table 3 showed comparison of uptake increase in the same hand from the time of 50 CPM to 310 seconds. The uptake increase in cold exposed hand of Raynaud’s group was 16.093 compared with 29.850 of normal control group. The increase in room air exposed hand of Raynaud’s group was 6.750 and that of normal control was 11.587, There was no significant, statistical difference between both groups in cold exposed and room air hand, respectively.
Table 4 presented the data in 10 patients of Raynaud’s before and after 2 weeks of medical therapy. In 4 patients of scleroderma, initial slope ratio before treatment was 0.428±0.104 (mean±SD) and after treatment was 0.630±0.168. The change of initial slope ratio between before and after treatment indicated improved blood flow rate in cold hands after 2 week therapy. When compared with 310 sec. cumulative digital blood flow, we found that there was somewhat improvement in digital blood flow. The 310 cumulative digital blood flow ratio was 0.440±0.073 before treatment and 0.651±0.082 after treatment.
In 4 patients of scleroderma, the ratio of activity in the cold exposed hand to room air exposed hand, 20 minutes after cold exposure changed from 0.545±0.128 (mean±SD) before treatment to 0.487±0.195 after treatment, which indicated no significant statistical difference. When comparing with 310 sec. cumulative digital blood flow ratio, we found that there was minimal change in digital blood flow.
When comparing with 310 sec. cumulative digital blood flow ratio, we found that there was minimal change in digital blood flow. The 310 sec. digital blood flow ratio was 0.440±0.073 before treatment and 0.651±0.082 after treatment. The similar result was noted in the patient of polymyositis. But in one case of MCTD, there was considerable improvement in digital blood flow after 2 weeks therapy as the value of 0.668 changed into 1.000 in the ratio of activity in cold exposed hand to room air expsed hand, 20 min. after cold exposure and 0.398 into 0.744 in 310 sec. cumulative digited blood flow ratio. We observed a similar degree of digital blood flow improvement in 1 patient of Behcet’s syndrome and 1 patient of SLE.
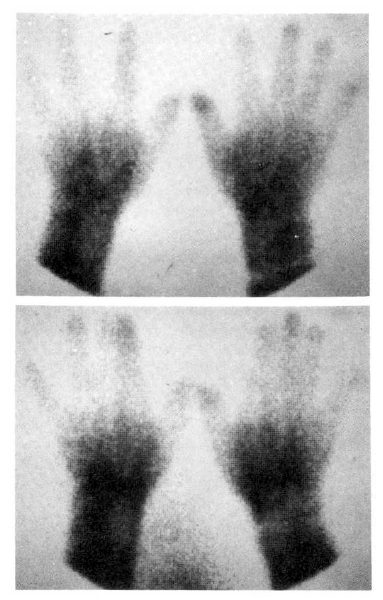
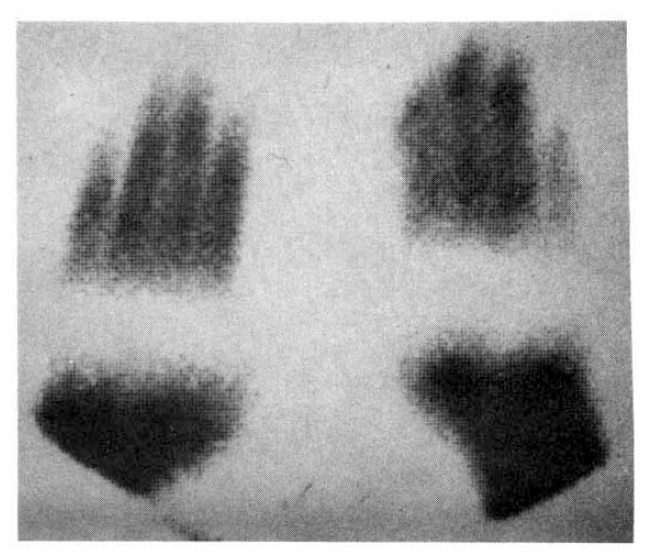
Fig. 5 showed a static image of scleroderma patient before treatment and decreased blood flow of right third, fourth and fifth fingers below the metacarpal area. Fig. 6 showed time activity curves before and after treatment in the same scleroderma patient in Fig. 5. The top panel was a time activity curve of pre-treatment and the low panel was a curve of post-treatment. We could know minimal improvement in blood flow even after the treatment. The static image of MCTD patient was in Fig. 7. The upper panel showed markedly decreased radionuclide uptake in left middle finger. Shown in the lower panel, the uptake in left middle finger in the same patient increased to some extent after 2 weeks of therapy. Fig. 8 demonstrated the same MCTD patient’s time activity curve in which the digital blood flow was much better after treatment. We could see reduced blood flow of right little finger in a localized scleroderma patient (Fig. 9).
DISCUSSION
Our knowledge of the pathophysiology, diagnosis and the treatment of Raynaud’s phenomenon has increased markeldy since the original description of the entity by Maurice Raynaud in 1862. However, Raynaud’s phenomenon remains a riddle despite over 125 years of research. In 1923, Sir Thomas Lewis21) first showed that the digital vessels in patients with Raynaud’s attacks are abnormally responsive to cold even in the absence of sympathetic innnervation. Lots of tests to evaluated the finger blood flow has been done until now. But patient history is still though to be the most reliable method of diagnosis. Several labortory tests, including immunologic tests and radiograph, should be included. Nerve conduction studies are also needed to exclude entrapment syndrome.
There were additional laboratory techniques for diagnostic accuracy. Porter et al22) used a thermocouple to measure the changes in skin temperature of the fifth finger after ice water immersion for 20 seconds. In the 30 controls, recovery averaged 10 minutes, wheras in 23 patients with Raynaud’s phenomenon the average was 30 minutes. Thermography, which measures thermal gradients along fingers, utilized a similar technique. The method of our radionuclide scitigraphy referred to the study of the thermocouple by Porter et al.
Plethysmography counted the increased volume of the digits because of the influx of blood. Sumner and Strandness16) found that 78 percent of 105 people with cold sensitivity produced a “peaked” and “peaked and obstructed” wave form. A similar form was seen in only 4 percent of control group. Plethysmography has been found to be a useful tool to follow the course of the treatment. Coffman and Cohen19) reported measurements of total digital flow plethysmographically and of capillary flow by radioisotope disappearance rate in Raynaud’s phenomenon.
Ultrasound can also be used to evaluate blood flow. Both ultrasound and plethysmography may be helpful in providing the location and degree of blockage in the vessels.
Using cryodynamic hand arteriogram, Rosch et al18) reported that 35 of 39 patients had basal digital vasospasm, whereas 34 of the same 39 patients also had organic obstruction involving primarily digital arteries. Porter et al23) reported that arteriogram seldom needed to establish the dignosis of the Raynaud’s. These authors utilized arteriography to evaluate a suspected surgically correctable proximal arterial lesions.
Laboratory testing for Raynaud’s phenomenon is only partially successful and may not be entirely producible, even after rigid standardization. Raynaud’s phenomenon continues to be a diagnosis based on a carefully taken history.
A wide variety of therapeutic management of Raynaud’s ayndrome are available but there are no definite and easy parameters to predict the outcome of therapeutic effect. There were no data to support that early dignosis and treatment of Raynaud’s influences outcome when associated with connetive disease.
We investigated digital finger blood flow with radionuclide scitigraphy after ice water exposure. MDP was used in this study by reason that the uptake of Tc-99m MDP is proportional to the amount of blood flow and most of Tc-99m MDP is circulated in blood during 5 minutes after intravenous administration24).
In comparison with 3 groups, we concluded that in 10 patients of Raynaud’s, 310 seconds cumulative digital blood flow ratio was significantly low and the initial slope ratio of cold exposed hand to room air exposed hand which was also low was the important parameter. There was no significance in the parameters such as the ratio of activity in cold exposed hand to room air exposed hand, and uptake increase in the same hand.
Of 8 patients with Raynaud’s phenomenon, 4 scleroderma and 1 multiple myeloma patients had minimal change in digital finger blood flow of cold exposed hands even after 2 weeks of therapy with vasodilators, calcium channel blockers or antiplatelet drugs. 1 MCTD, 1 Bechect’s syndrome, and 1 SLE patient had improved finger blood flow after therapy.
Here, the benefits of our simple clinical test to allow both an objective diagnosis and objective assessment of therapeutic response were apparent.
In summary, the method to evaluate finger blood flow with a radionuclide is a simple, ecomomical and objective assessment of the follow-up and therapeutic effects and gives an aid in the study of the pathophysiology of the Raynaud’s phenomenon.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





