 |
 |
| Korean J Intern Med > Volume 6(1); 1991 > Article |
|
Abstract
Cysticercosis, which has a worldwide distribution is found in man, who is usually infected by eating inadequately cooked pork or other contaminated food. Cysticercosis develops most commonly in the muscles and brain. Pulmonary involvement is very rare and also difficult to recognize because pulmonary lesions caused by the presence of cysticerci are difficult to discern from pulmonary infiltrates, because other parasitic infestations or tuberculosis, as well as metastatic lesions, produce similar chest X-ray findings and similar clinical symptoms.
We experienced a case of pulmonary cysticercosis confirmed at Gyeongsang National University Hospital by means of an open lung biopsy and treated successfully with praziquantel (50 mg/kg per day for 15 days).
This case seems to indicate that pulmonary cysticercosis should be considered as a diagnostic possibility in patients with nodular infiltrates in the lungs, especially in endemic areas, until such infiltrates are otherwise explained.
Cysticerci, the larval forms of Taenia solium, develop in the muscles of the pig, an intermediate host. Inadequately heated or raw pork containing adult worms is the main source of human infection.
Other sources of human infections with C. cellulosae occur by way of 1) ingestion of the eggs found on contaminated hands or in food, 2) self-contamination by people who have the adult worm in their intestines, and 3) internal autoinfection in which the eggs of the adult worm residing in the upper gastrointestinal tract are returned to the stomach by reverse peristalsis. The eggs are hatched in UGI to the embryos which penetrate the intestinal wall and are then carried along blood vessels to all parts of the body, where they soon become cysticerci. Man, in this case, becomes their intermediate host1,2).
Cysticerci most frequently invade the intermuscular and subcutaneous tissues; next, the eye and then the brain. They may also invade the heart, liver, abdominal cavity, kidneys, and adrenals, as well as the lungs3).
Pulmonary cysticercosis, however, is very rare. It is so rare that, worldwide, only a few cases have ever been reported, with none in Korea. Among those few cases reported in other areas, fewer cases still have been confirmed by direct examination of the lung tissues.
This report, then, together with relevant literature, cites another one of the few documented cases of pulmonary cysticercosis and further cites the only case of pulmonary cysticercosis ever documented or reported in Korea.
A 65-year-old man was admitted to the hospital because of generalized weakness, coughing, and sputum production. Two months before his admission, weakness, sweating and anorexia had developed together with an intermittent cough and whitish sputum production. His slowly worsening symptoms brought him to the hospital.
The patient had eaten raw pork several times 20 years ago, but there was no specific family history.
Vital signs were as follows: Temperature 36┬░, pulse 98/min, and respiration 20/min. The blood pressure was 110/75 mmHg.
On examination the patient appeared weak and chronically ill. Although consciousness was clear, his general appearance and nutritional condition were not good. His conjunctiva and cornea appeared normal. His lungs were clear without crackles or wheezing. S1 was normal and S2 was normally split. No murmur was heard. An abdominal examination disclosed mild tenderness to deep palpation in the right upper quadrant, without diminution of bowel sounds; the liver was palpated by four finger breadths. Neurological examinations were negative. There were multiple subcutaneous nodules in the upper extremities and axillas.
Laboratory data on admission were as follows: Hemoglobin and hematocrit were 13.9 g/dl and 41.8% on admission; white cell count was 8700 with 4% eosinophils; urine and stool specimens gave normal results; blood urea nitrogen 5 mg/dl; creatinine 1.3 mg/dl; total protein 8.1 g/dl; albumin 4.3 g/dl; and SGOT/GPT 42/32 u/dl. HBsAg and HBsAb were negative.
The stain for acid fast bacilli and cytologic examination of sputum did not reveal anything microscopically.
The pulmonary function test was within normal range; the FEV1 was 4.1, the FEV1/FVC was 87%.
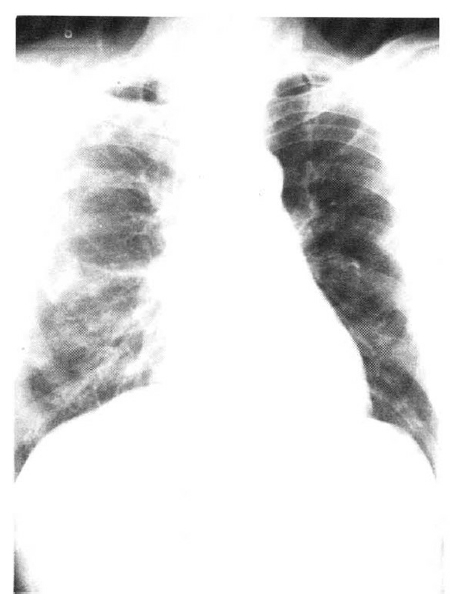
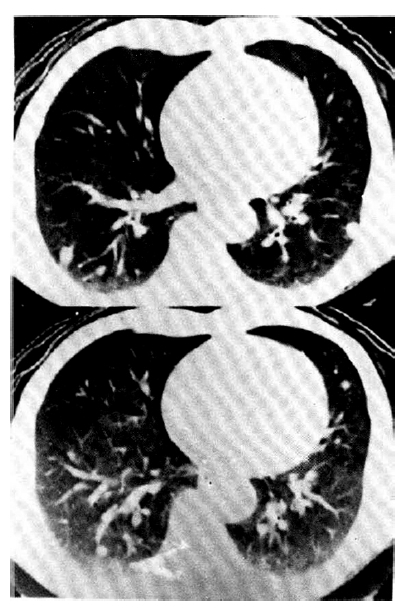
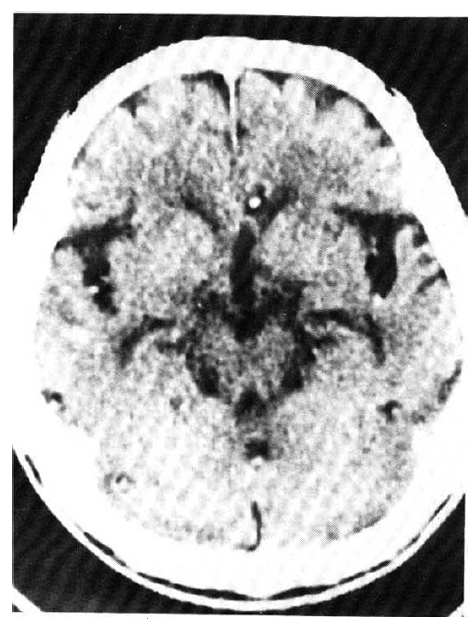
An X-ray film of the chest showed multiple nodular densities throughout both lower lung fields (Fig. 1). A computed tomographic scan of the brain and chest revealed multiple nodular and small calcified densities (Fig. 3, 4).
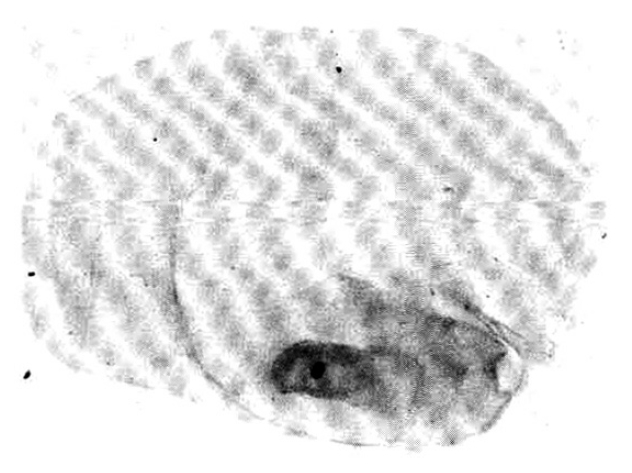
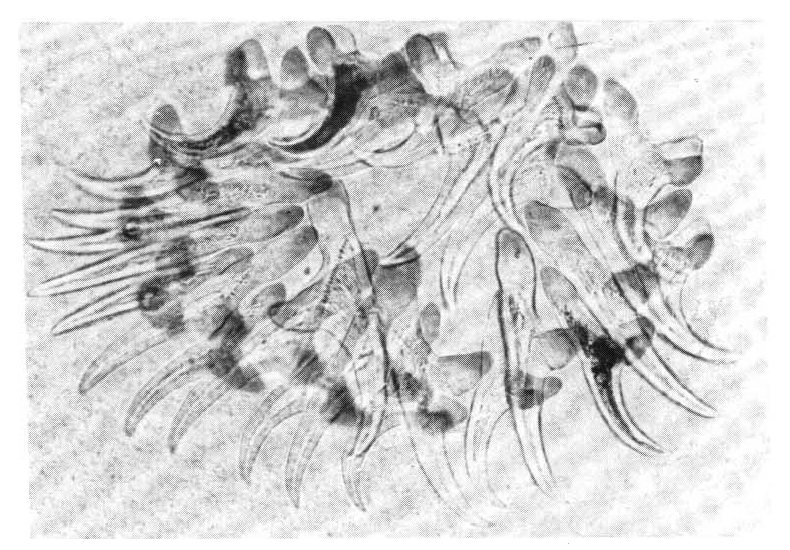
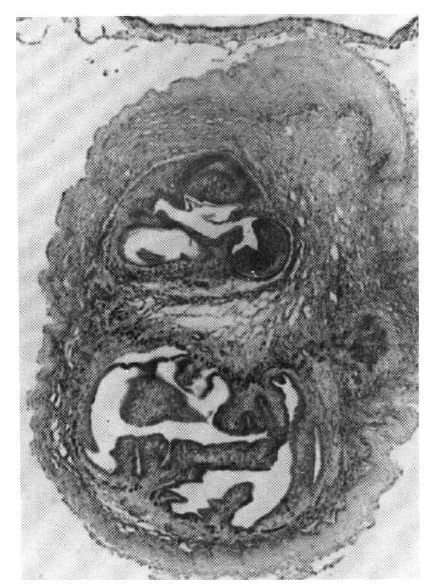
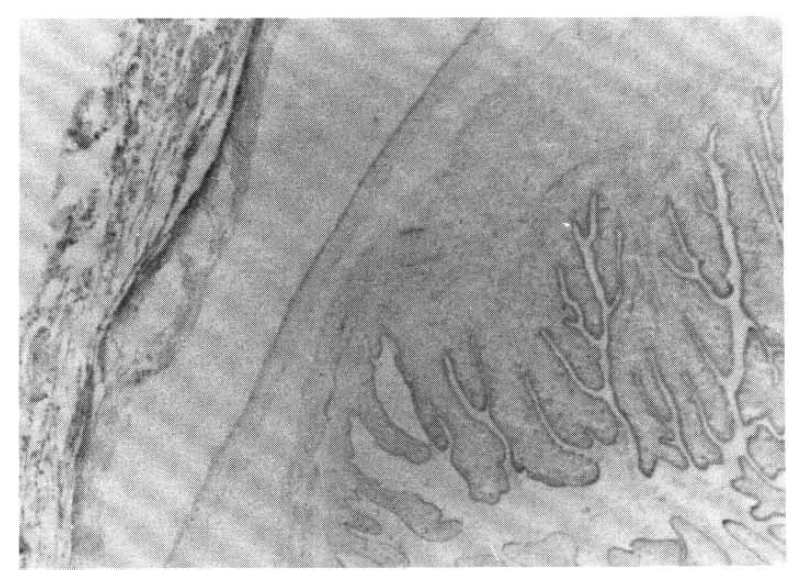
A biopsy of a subcutaneous nodule was done on the 4th hospital day, and upon microscopic examination, a bladder worm (Fig. 5)ŌĆöits hooks (Fig. 6) and parenchymatous portion with spinal canal and separated bladder of the cysticercus (Fig. 7)ŌĆöwas observed. On the 12 th hospital day, the presence of cysticercus was confirmed by an open lung biopsy (Fig. 8).
Afterward, the patient was treated with praziquantel 50 mg/kg/day for 15 days and discharged on the 18 th hospital day, even though he still reported some weakness, cough, and sputum production. One month after discharge, his symptoms began to improve. Six months after treatment, the subcutaneous nodules had decreased to half in size. An X-ray film of the chest revealed nearly normal conditions in the lungs(Fig. 2).
Taenia solium has been recognized from the time of Hippocrates but was never specifically differentiated from the beef tapeworm, T. saginata, until the time of Goseze (1782). Leukart (1856) first worked out the life cycle and demonstrated that the bladder worm in the tissues of the pig was in the larval stage and infective for man3).
Taenia solium has a world wide distribution but is most commonly found in the Soviet Union, Asia, Africa and in Central and South America4). The eggs of Taenia solium and Taenia saginata in Korea were found in 1.1% of the population, according to a broad parasite study done on 35,018 people in 19815). In 1986, the infection rate for the both parasites decreased to 0.27% (119 of 43,590 people)6). About 10% of those infected were thought to be infected with T. solium.
The eggs of Taenia solium are spherical or subspherical in shape, measure 31 to 43 um in diameter and cannot be microscopically distinguished from those of T. saginata. Cysticerci, the larval forms, are subspherical to ovoid, have milky white bladders with heads invaginated into the bladders, and are approximately 5 mm wide and 8 to 10 mm long. After human ingestion, the eggs develop in the stomach and intestine into embryos, which penetrate the intestinal mucosa, then circulate in the entire body through the venous system of the mesentery and are finally distributed in the muscles and other tissues where development into cysticerci takes place over a period of 60 to 70 days7).
The symptoms produced vary according to the location and number of cysticerci present. The fibers of the involved muscles atrophy and their function decreases. In the subcutaneous tissues, however, only a mild tissue reaction occurs, despite the swollen appearance of the protruded skin.
The clinical picture of brain cysticercosis varies according to the involved area of the brain cortex and can easily be mistaken for a brain tumor because of those symptoms which involve the central nervous system. If cysticerci develop in the ventricles of the brain, headache, nausea and vomiting occur due to increased intracranial pressure.
Precysticercus larvae lodged in the brain produce little disturbance during their life spans, but as soon as the larvae begin to lose vitality and parts of them die, dead larvae and their remnants are sensitized as foreign materials and evoke a great variety of brain symptoms, such as allergic reactions8,9).
When examples of pulmonary cysticercosis are found, cysticerci may be assumed to have also invaded many other organs like the brain, subcutaneous tissues and muscles of humans or animals as well. The radiological appearance of cysticercosis in the lungs cannot be differentiated from other parasitic infections, e.g., ecchinococcosis, pentastomiasis, paragonimiasis, and histoplasmosis, or other conditions such as tuberculosis, alveolar carcinoma and metastases. This is due to the varying reactions of the lung tissues and to the difference in size of the larvae.
The reason for the rarity of lung involvement is probably explained by the life cycle of the Taenia solium parasite. Humans may serve as intermediate hosts for the adult larvae which favor muscle and brain tissue to complete their life cycle. Another reason for the rarity of lung involvement may be that pulmonary lesions are overlooked because of the asymptomatic clinical features and because patients usually present with neurological symptoms10,11).
Although rare, pulmonary cysticercosis does occur; therefore it should not be overlooked in differential diagnosis of multiple lung opacities just because they are relatively frequent in Korea. Like brain cysticercosis, which is treated with praziquantel (50 mg/kg/day) for 15 days12), pulmonary cysticercosis, in this case, was treated completely by the same dose, even though no regimen has yet been established.
REFERENCES
1. Cho Soo Ho, Moon Choong Bae, Yearn Choi Byung. Clinical analysis of CNS Cysticercosis. The Yeungnam Univ Med J 1:25. 1984.

2. Byong Seol Seo. Clinical parasitology. 2nd ed. 279. Seoul: II Cho Kak, 1983.
3. Beaver PC, Jung RC, Cupp EW. Clinical parasitology. 9th ed. 513ŌĆō519Philadelphia: Led and Febiger, 1981.
4. Pawlowski E, Schulz MG. Taenia and cysticercosis (Taenia saginata) Adv. Parasitol 10:280ŌĆō2951972.
5. Prevalence of intestinal parasitic infection in Korea. The 3rd report p 25. The ministry of health and social affairs 1981.
6. Prevalence of intestinal parasitic infection in Korea. The 4th report p 25. The ministry of health and social affairs 1986.
7. Salis J. The morphology and pathogenicity of the bladder worm 81ŌĆō104Praqul. academia(publishing house of the Czechoslovakia academy of science). 1970.
8. Edwin R. Bickerstaff: Cerebral cysticercosis common but unfamiliar manifestations. British Medical Journal 30:1055. 1955.
9. Veliath Andrew J, Ratnakar C, Thakur LC. Cysticercosis in south India. Journal of topical medicine and hygiene 88:25. 1985.
10. Lennon Evan, Longo PG. The recognition of cysticercosis on chest radiographs. Aust Radio 10:14. 1966.

12. Ramsey Paul G, Plorde James J. Cestode infections HarrisonŌĆÖs principles of internal medicine. In: Braunwald Eugene, Isselbacher Kurt J, Petersdorf Robert G, Wilson Jean D, Martin Joseph B, Fauci Anthony S, eds. 11th ed. 825. New York: McGraw-Hill Book Company, 1987.
-
METRICS

- Related articles
-
Bone metastasis in pulmonary sclerosing hemangioma2015 November;30(6)
A case of ampullary gangliocytic paraganglioma2014 May;29(3)
A case of scar sarcoidosis2008 December;23(4)
A Case of Pulmonary Vein Tumor Presenting as a Left Atrial Mass2007 March;22(1)
A Case of Primary Intestinal Lymphangiectasia1993 January;8(1)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


