INTRODUCTION
In recent years, ischemic heart disease has become a major cause of death and disability. Consequently, a major research effort has been devoted to salvage ischemic myocardium. The evaluation of these experimental approaches requires a direct and specific method that can detect primary changes resulting from pathology. However, there are few diagnostic agents that are truly tissue specific, and many of the methods used in cardiovascular diagnosis depend on indirect rather than primary changes.
Radiolabeled substances sequestered by the necrotic myocardium may provide a more direct method in the detection and quantitation of myocardial necrosis. Tc-99m pyrophosphate has been one of the most widely used radiopharmaceuticals, and pyrophosphate uptake appears to indicate irreversible myocardial injury1ŌĆō4). However, controversies still exist regarding the extent of the uptake in the ischemic nonnecrotic myocardium5ŌĆō7), and since the local concentration is affected by the blood flow, the uptake tends to be related to the extent of the reduction in blood flow8ŌĆō10). Since 1976 when Dr. Khaw and his colleagues demonstrated that the uptake of antimyosin is specific for myocardial necrosis, radiolabeled antimyosin antibody has been shown to be useful in the detection of myocardial injury and is commonly used in the diagnosis of myocardial infarction11ŌĆō23).
Previous studies involving these two infarct avid radiopharmaceuticals frequently utilized the persistent coronary occlusion model8,9,12). However, radiopharmaceutical distribution is restricted by blood flow. Therefore, a reflow model is more appropriate for the comparison of the distribution of the infarct localizing agents. We produced an experimental myocardial infarction by occlusion of the left anterior descending coronary artery for 90 minutes followed by reperfusion. The uptakes of In-111 antimyosin and Tc-99m pyrophosphate were analyzed.
METHODS AND MATERIALS
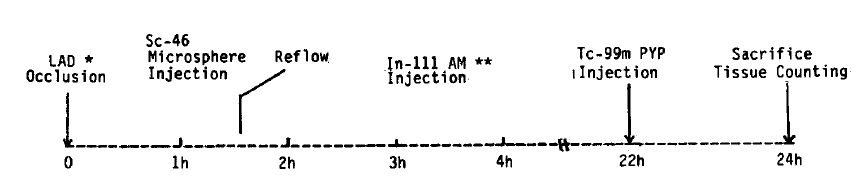
Adult mongrel dogs were anesthetized with intravenous sodium pentobarbital (30 mg/kg). Under sterile conditions, a left thoracotomy was performed through the fifth intercostal space, and the heart was isolated in a pericardial cradle. The left anterior descending coronary artery (LAD) was carefully freed by dissection and ligated at a level just distal to the first diagonal branch. Complete coronary occlusion was confirmed by visual inspection of the artery and by the appearance of epicardial cyanosis over the anterolateral left ventricle (Fig. 1).
Just before the release of ligation, the relative regional myocardial blood flow was measured by atrial administration of Sc-46 labeled microspheres (Du Pont). Microspheres were obtained as 1 mCi of radionuclide suspended in 10 ml of 10% dextran. Approximately 2 ├Ś 106 microspheres were injected into the atrium as a 1 ml of bolus and subsequently flushed with 8 ml of warm isotonic saline. After 90 minutes of occlusion, the ligature was released, and reflow was confirmed by the development of reactive hyperemia in the previous cyanotic area. At two hours after reperfusion, 0.5 mCi of In-111 antimyosin Fab (Centocor, Inc., USA) was administered intravenously. Anticardiac myosin antibody was a Fab fragment of the monoclonal antibody, and 0.25 mg of Fab was labeled with 1 mCi of In-111 chloride. Labeling efficiency was measured by Sephadex G-50 column chromatography. Samples with >85% incorporation of In-111 were considered usable. Twenty-two hours after coronary occlusion, 5ŌĆō10 mCi of Tc-99m pyrophosphate was injected intravenously, and the animals were sacrificed two hours later.
Immediately after the sacrifice, the heart was excised and the left ventricle was dissected free. Transverse left ventricular slices were cut with a blade perpendicular to the apex of the base axis and were approximately 10 mm thick. Transmural samples were sectioned from the slices. In four dogs which survived throughout the experiment, 80 to 100 samples per dog were taken from the periphery and the center of the area supplied by the LAD and normal nonischemic posterior myocardium. Each sample was divided into epicardial and endocardial halves, weighed and counted in a well scintillation counter utilizing differential spectrometry. Sample activity was obtained as counts per minute per gram of tissue.
Relative subendocardial and subepicardial uptakes of Tc-99m pyrophosphate and In-111 antimyosin Fab were calculated as the ratio of uptake in the infarcted regions to that present in the nonischemic posterior left ventricular subendocardium and subepicardium, respectively. Average, weight-corrected normal values for each subendocardial and subepicardial myocardium were calculated by averaging the values from 10 to 20 samples obtained from the posterior left ventricular myocardium. The relative myocardial (subendocardium and subepicardium) blood flow was also expressed as a percent of the nonischemic posterior subendocardial and subepicardial blood flow which were determined by the uptake of Sc-46 labeled microsphere.
The statistical significance was evaluated using an unpaired t test, and a linear regression analysis relating the relative antimyosin and pyrophosphate uptake to regional blood flow was obtained.
RESULTS
1. Localization of Tc-99m Pyrophosphate and In-111 Antimyosin Fab in Infarcted Subendocardial Myocardium
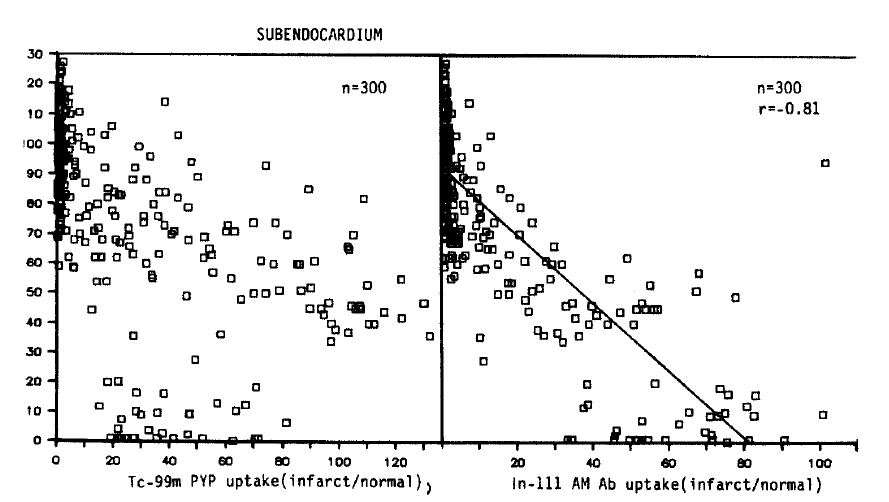
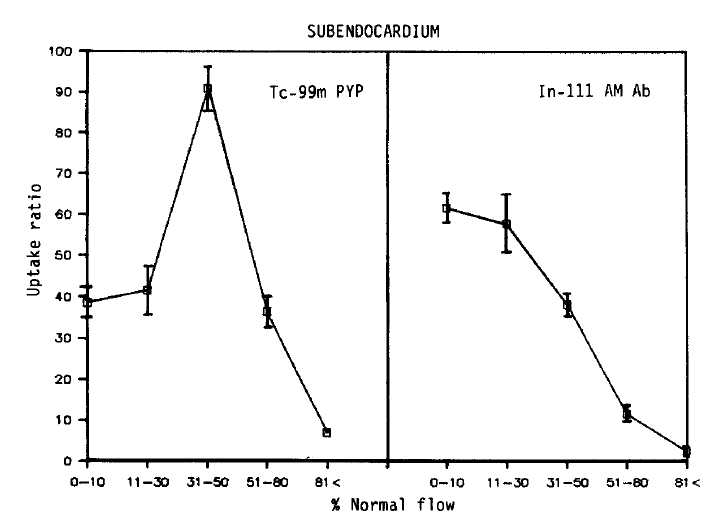
The relative localization of Tc-99m pyrophosphate and In-111 antimyosin in relation to the regional subendocardial blood flow in all subendocardial sample is shown in Figures 2 and 3.
No simple linear relationship existed between the blood flow and Tc-99 m pyrophosphate localization. Maximum uptake (90.8 ┬▒ 6.0, Mean ┬▒ SEM) was observed in areas of moderate flow reduction (31ŌĆō50% of normal). In myocardial samples where the flow was reduced to 0ŌĆō10% or 11ŌĆō30% of the normal, the relative uptake was 38.5 ┬▒ 3.7 and 41.6 ┬▒ 6.2, which were similar to the uptake of the areas of 51ŌĆō80% flow (36.3 ┬▒ 3.7). At a regional blood flow greater than 81%, significant uptake of pyrophosphate persisted (7.1 ┬▒ 1.2).
On the other hand, the relationship between the flow-related microsphere distribution and antimyosin localization was inverse linear (r = ŌłÆ0.81). The uptake was maximal in areas of 0ŌĆō10% of the normal blood flow (61.7 ┬▒ 3.5). The relative localization was 57.8 ┬▒ 7.0 in specimens where the flow was recued to 11ŌĆō30% of the normal, and 38.2 ┬▒ 3.1 in regions where the relative flow distribution was 31ŌĆō50% of normal. In areas where the flow was only mildly (51ŌĆō80% of the normal) or slightly (>81% of normal) reduced, the antimyosin uptake progressively diminished to 11.6 ┬▒ 1.7, 2.6 ┬▒ 0.7, respectively.
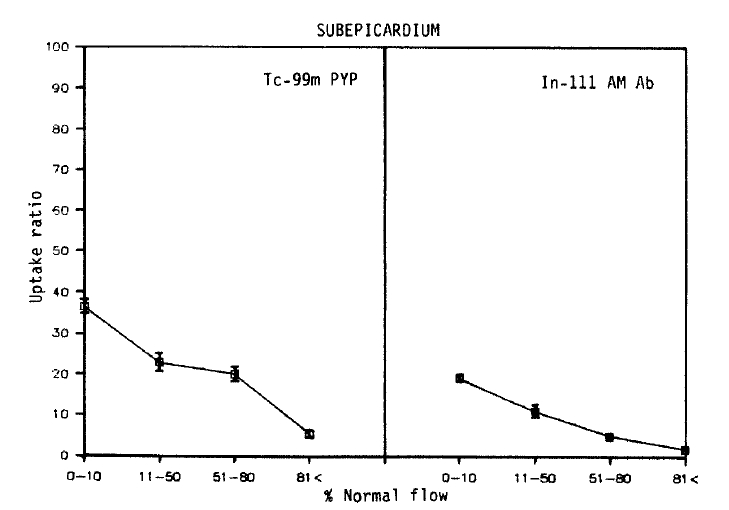
2. Localization of Tc-99m Pyrophosphate and In-111 Antimyosin Fab in Infarcted Subepicardial Myocardium
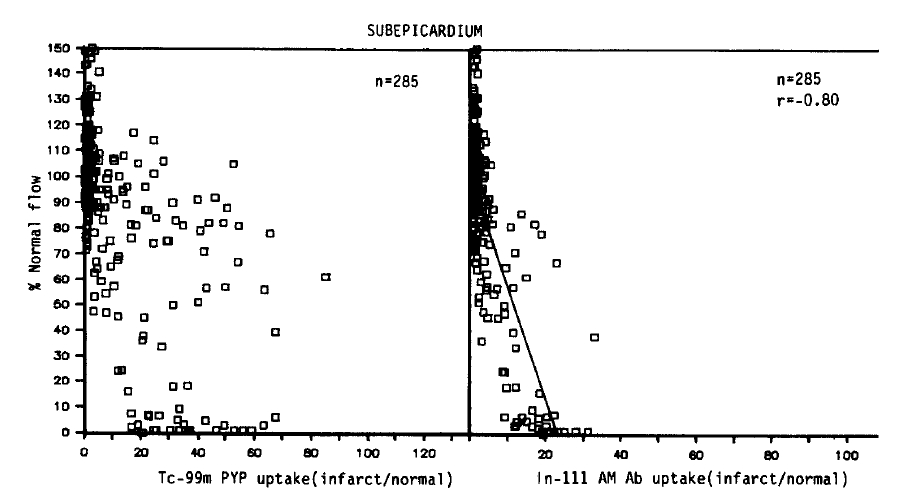
As shown in Figures 4 and 5, qualitatively similar trends were observed in all subepicardial myocardial samples. Again, no simple corrleation between the blood flow and Tc-99m pyrophosphate uptake was found. However, in contrast with the data obtained from the subendocardial myocardium, the maximum uptake (36.3 ┬▒ 3.0) was localized to areas of severe flow reduction (0ŌĆō10% of normal), and the mean relative uptakes were lower than those of the subendocardium. In the myocardial samples where the flow was reduced to 11ŌĆō50% or >81% of the normal flow, relative localization diminished to 22.9 ┬▒ 4.3, 20.1 ┬▒ 4.0 and 5.4┬▒0.7.
As seen in the subendocardial samples, an inverse linear relationship was observed between the blood flow and the antimyosin localization (r = ŌłÆ0.80). But the slope was deep, and comparable decreases in the antimyosin concentration were observed. The antimyosin uptake was the highest (19.2┬▒1.0) in areas of the lowest flow area (0ŌĆō10%) and progressively decreased to 11.0┬▒2.0 in areas of 11ŌĆō50%, 5.0┬▒1.0 in 51ŌĆō80% flow and 1.9┬▒0.1 where the regional flow was >81% of the normal.
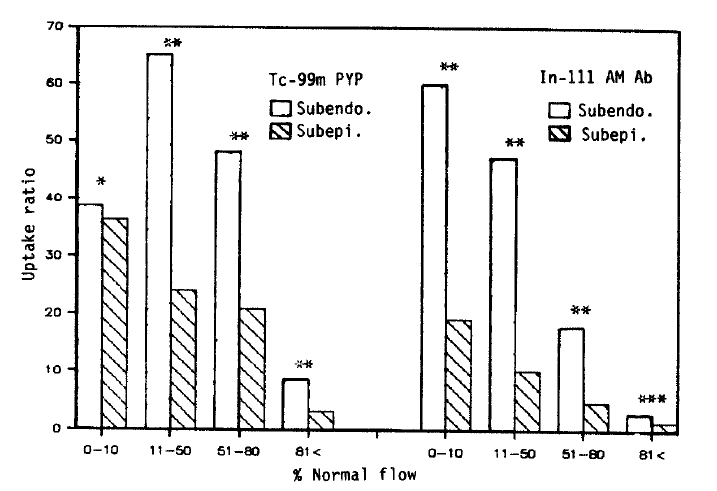
3. Comparison Between Subendocardial and Subepicardial Distribution of Tc-99m Pyrophosphate and In-111 Antimyosin Fab
To compare the relative localization of radiopharmaceuticals between the subendocardium and subepicardium, the relative myocardial blood flow was recalculated as a percent of the nonischemic posterior myocardial blood flow, which was obtained by averaging both normal subendocardial and subepicardial values.
Except for the Tc-99m pyrophosphate uptake in the severe flow reduction area, the subendocardial uptake of both Tc-99m pyrophosphate and In-111 antimyosin was significantly greater than that of the subepicardium. This difference was observed between the subendocardial and subepicardial layers where the degree of blood flow reduction was similar (Fig. 6).
DISCUSSION
The usefulness of radiolabeled anticardiac myosin antibodies has been demonstrated in the detection and quantitation of myocardial necrosis24ŌĆō26). It is believed that cell membrane integrity is disrupted after myocardial injury, and this permits the entry of the antibodies that then bind cardiac myosin. Recently, its efficacy in the diagnosis of myocarditis and human cardiac transplant rejection has been advocated27ŌĆō30). The histopathological demonstration of myocyte necrosis is critical in the diagnosis of these disease. We also demonstrated the value of antimyosin antibody as a tool in the evaluation of myocardial necrosis31ŌĆō32).
Our data demonstrated an excellent correlation between the myocardial In-111 antimyosin uptake and myocardial necrosis as assessed by the regional blood flow. There was an inverse relationship between the antimyosin uptake and the myocardial flow, and maximum localization occurred in the areas of the lowest blood flow. Another fact was that in areas of slight-to-mild flow reduction, the antimyosin uptake was not high as that in areas where the flow was severely impaired. These results suggests that antimyosin uptake is specific for irreversibly ischemic myocardium and can be used as a quantitative tool in vitro analysis of myocardial necrosis. Our results were similar to the data of previous studies from the persistent occlusion model12). This may be due to the long half-life of antimyosin. Therefore, there is sufficient time for to accumulate in areas of low flow even in the persistent occlusion of the coronary artery.
We used the relative myocardial blood flow as an index of myocardial necrosis. Obviously it would be more appropriate to study histopathological examination or histochemical staining, such as triphenyl tetrazolium chloride staining, to determine the extent of tissue necrosis33). However the myocardial oxygen supply is closely related to the amount of myocardial blood flow, and considering the differences of oxygen demands between the subendocardial and subepicardial layers, we divided the myocardial samples into two groups and analyzed radiopharmaceutical localization in both groups separately.
Reperfusion is now an important modality for treating myocardial ischemia produced by coronary occlusion. Therefore, understanding the pathophysiologic correlates of myocardial radionuclide uptake may provide useful information in the evaluation of myocardial necrosis. Another reason we selected the reflow model was that isotope distribution is not influenced by blood flow. Jennings et al. demonstrated that irreversible myocardial damage occurred between 20 to 60 minutes of severe ischemia, and 60 to 180 minutes of moderate ischemia34). In another study by Kloner et al., both qualitative and quantitative measurements of blood flow distribution failed to demonstrate the perfusion defects characteristic of ŌĆ£no-reflow,ŌĆØ and no marked defects in reflow had been observed until 90 minutes of severe ischmia elapsed35). In this study the duration of coronary occlusion was 90 minutes in order to produce an adequate amount of myocardial necrosis for analysis and not to induce microvascular damage which would impede inflow into the ischemic zone.
In contrast with antimyosin localization, the pyrophosphate uptake did not show a discrete correlation. Maximum subendocardial uptake occurred at a regional blood flow of 31ŌĆō50% of the normal. Here we cannot exclude the possibility of ŌĆ£no-reflowŌĆØ phenomenon. In fact, subendocardial distibution of pyrophosphate may be explained by no-reflow in a severely ischemic area. But in subepicardial layers where microvascular injury was not expected to be present36,37), no simple linear relation existed. In addition, there was significant myocardial uptake in regions where the flow was minimally reduced (>81%). This may suggest pyrophosphate uptake in ischemic myocardium. On the other hand, these areas may contain small populations of infarcted myocardial cells interspersed with normal cells.
The transmural distribution of Tc-99m pyrophosphate in Fig. 6 is different from the results obtained from the persistent occlusion model8,12). Apparently the delivery of pyrophosphate into the more intense necrotic subendocardium was not restricted by coronary occlusion in our model. Parkey et al. also reported that in the reperfused infarct model, the pyrophosphate uptake is not restricted to the periphery10). Another interesting finding is that the difference between the subendocardial and subepicardial uptake of these two radiopharmaceuticals was observed in areas where the degree of blood flow reduction was similar. A greater degree of metabolic demand of the subendocardial myocardium may be responsible for this difference.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





