 |
 |
| Korean J Intern Med > Volume 4(1); 1989 > Article |
|
Abstract
To investigate the status of the Na+ concentrations [Na+]i, K+ concentrations [K+]i and ionic fluxes in red cells of human subjects with abnormal thyroid function, we measured the Na+-K+ pump activity as well as Na+-K+ contransport (CoT), Na+-Li+ countertransport (CTT) and Na+ passive permeability in erythrocytes of 37 normal subjects, 19 untreated hyperthyroid patients, 12 treated hyperthyroid patients and 9 hypothyroid patients with T4 replacement.
The mean [Na+]i value in the untreated hyperthyroidism group was significantly higher than that in the normal subjects (p<0.5), but not significantly different from that in the treated hyperthyroidism group. The mean [Na+]i value in the hypothyroidism with T4 replacement group, however, was significantly lower than that in the normal group (p<.01). We did not find any significant difference of [K+]i in comparing each group. It was found that the Na+-K+ pump activity in erythrocytes was significantly increased in untreated hyperthyroidism (mean; 23.4% above control, p<10ŌłÆ5), but there was no significant difference in treated hyperthyroidism and hypothyroid patients with T4 replacement. The rate constant for ouabain-sensitive Na+ efflux in the hypothyroidism with T4 replacement group was markedly higher than that in normal subjects (p<.01), but not significantly different in the untreated hyperthyroidism group. We observed a significant increase of the Na+ CoT value in the patients with untreated hyperthyroidism as campared with that of the normal subjects (p<.05), but there was no significant difference in the patients treated for hyperthyroidism and the hypothyroidism with T4 replacement group. However, the rate constant for Na+-CoT in the patients with hypothyroidism with T4 replacement was significantly higher than that in normal subjects (p<.05). We observed a marked decrease of Na+-Li+CTT value in the patients with untreated hyperthyroidism versus that in the normal group (p<.01). Passive Na+ permeability in the patients with untreated hyperthyroidism was markedly increased (p<.05), and was markedly decreased in the patients with hypothyroidism with T4 replacement compared to normal subjects (p<.01). It can be concluded from these studies that an increase in Na+-K+ pump activity in the patients untreated for hyperthyroidism might then be regarded as a secondary adaptive cellular response to higher [Na+]i values due to enhanced passive Na+ permeability, rather than a direct effect of the thyroid hormone.
Stimulation of Na+-K+ pump activity by the thyroid hormone in several tissues, including liver, kidney, skeletal muscle and heart has been demonstrated in a wide variety of experimental studies1ŌĆō10) and has been proposed as a major mediator, i.e. metabolic pacemaker of thyroid thermogenesis11ŌĆō13). However, despite these observations, the mechanism by which the enhancement of Na+-K+ pump activity is mediated by the thyroid hormone has not been resolved. Although it has been postulated that the effect of thyroid hormone on active cation transport is mediated by direct stimulation of sodium-potassium pump unit synthesis5,7,12), it is not clear how an induction of additional Na+-K+ pump units per se could explain a steady state enhancement of active sodium and potassium transport in the absence of an augementation of passive fluxes of cations opposite to the direction of their active transport.14) Recent evidence does in fact exist to suggest that thyroid hormone increases monovalent cation permeability in isolated rat skeletal muscle15) and perfused rat liver.5,14)
In contrast to the above animal data, little information on the state of the Na+-K+ pump in human patients with altered thyroid function is available; moreover, the available data on human patients appears contradictory to the results in animals. Thus, Cole et al. reported that the Na+-K+ ATPase activity in human erythrocyte membranes was reduced in hyperthyroidism and this finding was consistent with the increase intracellular sodium concentration [Na+]i.16) To further examine this apparent change in the cation transport in red blood cells, we have measured the Na+-K+ pump activity as well as Na+-K+ cotransport (CoT), Na+-Li+ countertransport (CTT) and Na+ passive permeability in patients with hyper- and hypothyroidism.
Nineteen untreated hyperthyroid patients, twelve treated hyperthyroid patients and nine hypothyroid patients with T4 replacement were recruited for this study; the majority were outpatients from the Endocrine Clinic of Kangnam St. MaryŌĆÖs Hospital. The relevant clinical and thyroid hormone data for each group are shown in Table 1. At the time of the initial measurements, untreated hyperthyroid subjects had been hyperthyroid status for at least 4 weeks, as judged from their clinical history and available laboratory data. The untreated hyperthyroid subjects were not any specific antithyroid drug or other medication, and in particular, no patient was being treated with agents such as corticosteroids which are known to affect the Na+-K+ pump activity. The treated hyperthyroid subjects had been managed with antithyroid drugs for at least 4 weeks. The clinical diagnosis of hypothyroidism was confirmed by the measurement of serum T3, T4 and TSH using standard radioimmunoassays. All of the hypothyroid subjects were managed with T4 replacement.
Thirty-seven control subjects were recruited from the medical student population, nursing personnel and doctors, all of whom were clinically and hormonally euthyroid. The mean age of the normal subjects was 30.2 (range; 24ŌĆō37 yrs), somewhat younger than that of the untreated hyperthyroid and hypothyroid subjects. In addition, there was approximately equal representation by males and females, in contrast to the preponderance of males in each study group. However, we were unable to detect any age or sex-related differences in the Na+-K+ pump measurement in our control population (Table 1).
Venous blood (10 ml) collected in heparinized tubes was centrifuged at 1,750 g for 10 min. The plasma and buffy coat were also aspirated. RBC were then washed twice with isotonic MgCl2 (110 mM). All steps were carried out at 4┬░C.
Cells were washed 3 times with 10 volumes of ice-cold isotonic MgCl2 solution. After mixing, duplicate hematocrit determinations were made of this suspension. Then 100 ╬╝l were diluted 1:50 in deionized water. The concentration of Na+ and K+ of the hemolysate was measured on a Perkin Elmer model 2380 Atomic Absorption spectrophotometer, and expressed as mmol/l of packed red cells by dividing the ionic concentration by the hematocrit and multiplying by the dilution factor.
Simultaneous measurements of the Na+-K+ pump, Na+-K+ CoT, Na+-Li+ CTT, and ouabain-and bumetanide-resistant Na+ and K+ fluxes (passive Na+ and K+ permeability) were made according to the modified method of Garay et al.17). Washed red cells were resuspended in cold Mg++ sucrose medium (mM): 75 MgCl2, 85 sucrose, 10 MOPS-Tris (pH 7.4 at 37┬░C) and 10 glucose, to a hematocrit of 20ŌĆō25%. A portion of the cell suspension was added (final hematocrit 4ŌĆō5%) to different cold solutions containing buffered Mg++ sucrose plus the following additions (mM): (1) 2 KCl, (2) 0.1 ouabain, (3) 0.1 ouabain plus 0.02 bumetanide, and (4) 10 LiCl, 0.02 bumetanide, and 0.1 ouabain. At time 0, the tubes were transferred to a 37┬░C bath for incubation. Sixty minutes after incubation, media 1,2,3, and 4 tubes were transferred to an ice bath for 1 min and then centrifuged at 1,750g for 5 min at 4┬░C. The supernatant was carefully removed, and the Na+ and K+ concentrations were measured by atomic absorption spectrophotometry, using standards prepared in the appropriate Mg++ sucrose media.
Na+ and K+ effuxes were computed using the following equation:
D Cat. (╬╝mol/l supernatant) is the difference between the external cation concentration (Na+ or K+) after incubation at 37┬░C and that at 0 time. The Na+-K+ pump activity was calculated by subtracting the Na+ efflux in the presence (medium 2) from that in the absence (medium 1) of ouabain. The Na+-K+ CoT fluxes were obtained by subtracting the Na+ efflux in the presence of ouabain plus bumetanide (medium 3) from that in the presence of ouabain alone (medium 2), while for the calculation of Na+-Li+ CTT, the Na+ efflux in medium 3 was subtracted from that in medium 4. The Na+ fluxes in medium 3 were taken as the ouabain-and bumetanide-resistant Na+ fluxes. The efflux rate constants for ouabain-sensitive and bumetanide-sensitive Na+ effluxes, and the influx rate constant for passive Na+ permeability expressed in hŌłÆ1, were calculated as the ratio of the respective fluxes to the erythrocyte Na+ content.
The mean [Na+]i value in the untreated hyperthyroidism group (15.3 ┬▒ 0.7 mmol/l RBC) was significantly higher than that in normal subjects (13.6 ┬▒ 0.4 mmol/l RBC) (p<.05), but not significantly different from that in the treated hyperthyroidism group (12.1 ┬▒ 0.8 mmol/l RBC). The mean [Na+]i value in the hypothyroidism with T4 replacement group (10.9 ┬▒ 0.7 mmol/l RBC), however, was statistically significantly lower than that in the control group (p<.01). Also, a statistically significant reduction of [Na+]i values in the treated hyperthyroidism group vs. that of those in untreated hyperthyroidism groups was noted (p<.01). The mean [K+]i values in each group were not significantly different from that of normal subjects (105.8 ┬▒ 2.1 mmol/l RBC) (Table 2, Fig 1).
Na+-K+ pump activity in the untreated hyperthyroidism group (2,083 ┬▒ 45 umol/l RBC/h) was significantly higher than that of the normal group (1,688 ┬▒ 52 umol/l RBC/h) (p<.10ŌłÆ5), but no significant difference was noted from that in the treated hyperthyroidism group and hypothyroidism with T4 replacement group. We observed a significant decrease of Na+-K+ pump activitiy in the patients being treated for hyperthyroidism compared to that of the untreated hyperthyroidism group (1,837 ┬▒ 73 vs. 2083 ┬▒ 45 umol/l RBC/h, p<.01). The rate constant for ouabain-sensitive Na+ efflux in the hypothyroidism with T4 replacement group (0.1738 ┬▒ 0.0095 hŌłÆ1) was markedly higher than that in the normal group (0.1359 ┬▒ 0.0059 hŌłÆ1) (p<.01). But there was no statistical significance in the rate constant between the normal and untreated hyperthyroidism groups (0.1396 ┬▒ 0.0054 hŌłÆ1). We observed a significant inverse correlation (r = ŌłÆ0.84, p<10ŌłÆ4) between the variation of the rate constant for the ouabain-sensitive Na+ efflux and [Na+]i, but not between the Na+-K+ pump activity and [Na+]i (r = 0.43, p = 0.07) (Table 2, Fig 2,3,4).
We observed a significant increase of the Na+ CoT value in the untreated hyperthyroidism group versus in the normal subjects (282 ┬▒ 20 vs. 192 ┬▒ 23 ╬╝mol/l RBC/h, p<.05), but no significant difference between the treated hyperthyroidism group and the hypothyroidism with T4 replacement group (266 ┬▒ 37 vs. 234 ┬▒ 38 ╬╝mol/l RBC/h). But, the rate constant for Na+ CoT in the hypothyroidism with T4 replacement group was significantly higher than that in the control group (0.0223 ┬▒ 0.0038 vs. 0.0134 ┬▒ 0.0022 hŌłÆ1, p<.05). There was no significant difference between each group in K+ CoT value (Table 2, Fig 5).
We observed a marked decrease of the Na+-Li+ CTT value in the untreated hyperthyroidism group vs. that of the normal group (80 ┬▒ 8 vs. 133 ┬▒ 12 ╬╝mol/l RBC/h, p<.01). But, there was no significant difference between the treated hyperthypoidism group and hypothyroidism with T4 replacement group (109 ┬▒ 21, 125 ┬▒ 21 ╬╝mol/l RBC/h), (Table 2, Fig 6).
Passive Na+ permeability in patients with untreated hyperthyroidism was markedly higher than that in normal subjects (339 ┬▒ 33 vs. 276 ┬▒ 11 ╬╝mol/l RBC/h, p<.05), whereas that in the hypothyroidism with T4 replacement group was markedly lower than that in normal subjects (198 ┬▒ 13 vs. 276 ┬▒ 11 ╬╝mol/l RBC/h, p<.01). However, the rate constant for passive Na+ permeability was not statistically significant between the groups (Table 2, Fig 7).
We have examined the interrelationships between erythrocyte sodium content and sodium transport in a group of healthy subjects and in groups of patients with abnormal thyroid hormones. The sodium content of the erythrocyte changes in a variety of acquired disorders including hypokalemia, digoxin toxicity, hyperthyroidism, hemorrhagic shock, chronic renal failure, and liver disease as well as in hereditary defects of the erythrocyte membrance.18) Boekelman, in 1958, first reported that patients with hyperthyroidism have an elevated concentration of sodium within their erythrocytes.19) Thereafter, many reports subsequently verified this observation and suggested that the determination of red cell sodium might prove useful as a measure of the peripheral action of thyroid hormone.18ŌĆō22) In this study, we have shown that untreated hyperthyroidism is associated with a significant increase in the intracellular sodium concentration (p<.05); however, treated hyperthyroidism does not bear the same results. In contrast to untreated hyperthyroidism, there was a significant decrease of [Na+]i in patients with treated hyperthyroidism (p<.01). What is the exact mechanism for the significant increase in [Na+]i in untreated hyperthyroidism?
A derangement of ion transport in altered thyroid function has been demonstrated in many tissues.1ŌĆō10) Sodium movements across human RBC membranes are mediated by several transport systems. The ouabain-sensitive Na+-K+ ATPase is the main mechanism maintaining the Na+ and K+ electrochemical gradients across RBC membranes against the passive Na+ and K+ diffusion. At least three additional transport systems have recently been described in human RBC membrans: (1) Na+-K+ CoT, (2) Na+-Li+ CTT, and (3) passive Na+ permeability.17)
Since Smith and Samuel (1970) reported that the increase in red cell sodium in patients with hyperthyroidism was associated with a decrease in the rate constant for active sodium efflux and significantly higher total active sodium efflux, the arguments for and against these results appeared.20) The thermogenic response to thyroid hormone has been observed in many tissues including heart, kidney, liver and skeletal muscle of rats. Recently, such a thermogenic response has been attributed to the stimulation of energy utilization resulting from the influence of this hormone on the sodium pump activity. Subsequently, thyroid hormone treatment has been shown to augment Na+-K+ ATPase activity, an enzyme system closely related to the sodium pump.1,2,7,12,13,23) Ismail-Beigi and Edelman recently proposed that augmentation of energy expended in active Na+ transport mediates a significant fraction of the thermogenic response to thyroid hormone. In rat liver and skeletal muscle, ouabain-sensitive or Na+-dependent respiration increased significantly in the transitions from the hypothyroid to the euthyroid and from the euthyroid to the hyperthyroid states induced by adminstration of T3.1) Asano et al.4) reported that administration of thyroid hormone induced enhancement of O2-dependent respiration in the liver, kidney and diaphragm of the rat, which was inhibited by cardiac glycoside. So the thermogenesis action of thyroid hormone might be mediated by Na+-K+ ATPase activity. The magnitude of the energy demands of the Na+ pump in the hyperthyroid state determines its importance as a metabolic pacemaker. In the rats studied by Lin and Akera, T3 treatment increased the [3H] ouabain binding site concentration in the liver, kidney, and skeletal muscle but failed to affect it in the brain.9) Although thyroid hormone increased Na+-K+ ATPase activity in many tissues, the result of erythrocyte Na+-K+ ATPase activity in hyperthyroid patients were controversial and little information on the state of the Na+K+ pump in human patients with altered thyroid function is available. However, in human subjects, activity of this enzyme, studied extensively in erythrocytes, has been found to be consistently reduced in the hyperthyoid state. Although Cole and Waddell (1976)16) tried to conclude from their studies that the decreased sodium efflux in the erythrocytes of patients with hyperthyroidism was associated with a decrease in Na+-K+ ATPase activity, the entry number was too small to draw this conclusion. In other studies, the number of Na+-K+ ATPase units in erythrocytes was found to be significantly reduced in patients with hyperthyroidism.18, 22, 24)
Nevertheless, in our study, Na+-K+ pump activity in the untreated hyperthyroidism group was markedly increased (p<.10ŌłÆ5), and in the treated hyperthyroidism group and the hypothyroidism with T4 replacement group, it tended to be higher than normal. Despite increased Na+-K+ pump activity in untreated hyperthyroidism, the rate constant for ouabain-sensitive Na+ efflux was not different from normal, but was significantly higher in the hypothyroidism with T4 replacement group than normal. There was no definite correlations between intracellular Na+ concentration and Na+-K+ pump activity. However, a significant inverse correlation between intracellular Na+ concentration and the rate constant for Na+-K+ pump activity was noted (r = ŌłÆ0.84, p<.10ŌłÆ4).
In this study, Na+-K+ CoT in the untreated hyperthyroid patients was higher than that in the normal patients, and tended to be higher, but not significantly in the treated hyperthyroid patients and the hypothyroidism with T4 replacement group. The exact mechamism for increased Na+-K+ CoT in the untreated hyperthyroid patients was uncertain. The observed increase in the Na+-K+ CoT, however, might be a secondary adaptive response of the cell to maintain normal intracellular ion concentration and transmembrane ion gradients in the face of this enhanced passive Na+ permeability. We found that Na+-Li+ CTT in the untreated hyperthyroid patients was markedly decreased; however, the mechanism was uncertain.
In our study, passive Na+ permeability in the untreated hyperthyroid patients was substantially higher than in the normal group, and tended to be lower in the treated hyperthyroidism and hypothyroidism with T4 group. Haber and Loeb have examined the effect of T3 treatment on the passive efflux of 42K+ from rat liver slices to determine whether an increase in permeability might play a role in the known enhancement of active monovalent cation transport of Na+-K+ ATPase activity induced by thyroid hormone. The magnitude and early onset of the effect of thyroid hormone on cellular potassium efflux raise the possibility that an increase in passive cation permeability may be a proximal event in the mediation of thyroid hormone action.14,15,25) Additional evidence for a thyroid hormone-induced increase in passive monovalent cation permeability has been provided by Folke and Sestoft5) in studies on perfused intact rat liver following T3 treatment in vivo.
As noted by Ismail-Beigi and Edelman, thyroid hormone-dependent activation of the Na+ pump could be a result of the following: (1) a rise in [Na+]i, perhaps as a result of increased membrane permeability to Na+ and consequent stimulation of the Na+ pump; (2) a change in the coupling ratio between the chemical reaction (i.e. ATP split) and transport rate, such that the rate of ATP hydrolysis is increased for a given rate of Na+ transport. As a result there would be a fall in the transmembrane Na+ and K+ gradients; (3) activation of mitochondrial ATP synthesis, resulting in stimulation of the Na+ pump by a local increase in ATP concentration; and (4) direct activation of the Na+ pump. In our study, the first (1) possibility, as either the sole or the predominant mechanism of the mediating role of Na+ transport in thyroid calorigenesis is supported in understanding the status of increased [Na+]i with the enhanced Na+-K+ pump activity in untreated hyperthyroidism.
Then, what is the exact mechanism for the pathologic alterations of cation movement in red blood cells? Abnormal RBC cation movements have been found in a wide variety of clinical states; i.e. (1) genetic abnormalities such as in hereditary spherocytosis; (2) precipitaion of cell contents such as in sickle cell disease, (3) membrane alterations induced by factors extrinsic to the RBC such as in hyperthyroidism and chronic uremia; and (4) formation of negatively charged ion pairs such as in hemolytic anemia.26)
All of the above findings suggest that untreated hyperthyroidism results in an early increase in membrane permeability with an augmentation of passive Na+ influx. The observed increase in the Na+-K+ pump might then be a secondary adaptive response of the cell to maintain normal intracellular ion concentration and transmembrane ion gradients in the face of the enhanced passive Na+ permeability. Although there is now general agreement that thyroid hormone stimulates sodium and potassium transport, the relative importance of this phenomenon in contributing to the thermogenic effect remains uncertain.
Notes
A preliminary report of this work was presented at the 38th annual meeting of the Korea Internal Medicine in Seoul. Korea, October, 1986. This work was supported in part by the Catholic Medical Center, clinical research funds.
Fig.┬Ā1.
Intracellular Na+, K+ concentration in normal controls and hyperthyroidism. *p<.05, **p<.01 vs control Mean┬▒S.E.M.
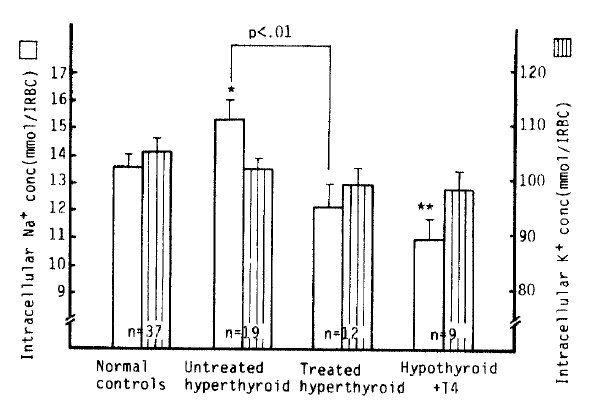
Fig.┬Ā2.
Na+-K+ pump and rate constant for Na+-K+ pump in normal controls and hyperthyroidism. *p<.01 **p<.001 vs control Mean┬▒S.E.M.
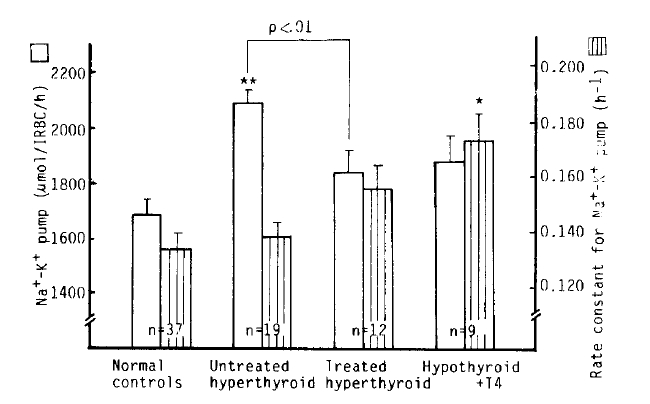
Fig.┬Ā3.
The relationship between the red cell sodium concentration and the rate constant for Na+-K+ pump from the cells in 19 patients with untreated hyperthyroidism.
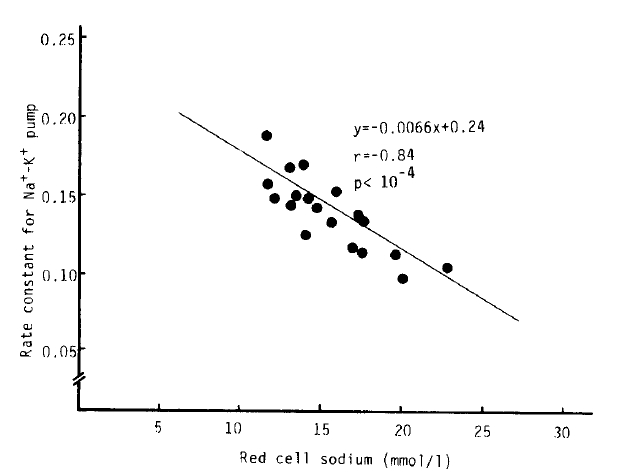
Fig.┬Ā4.
The relationship between the red cell sodium concentration and the Na+-K+ pump from the ceils in 19 patients with untreated hyperthyroidism.
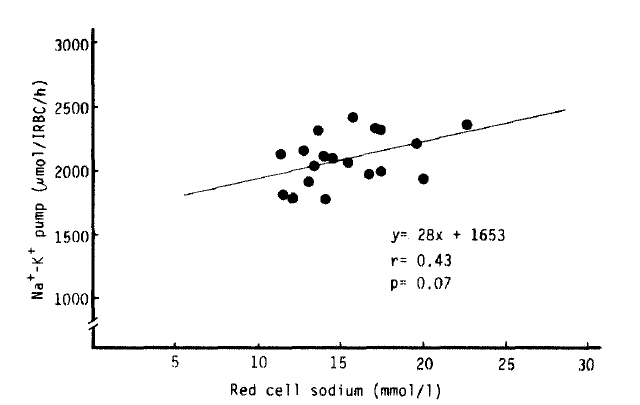
Fig.┬Ā5.
Na+ cotransport and rate constant for Na+ cotranspoert in normal controls and hyperthyroidism. *p<.05 vs control Mean┬▒S.EM.
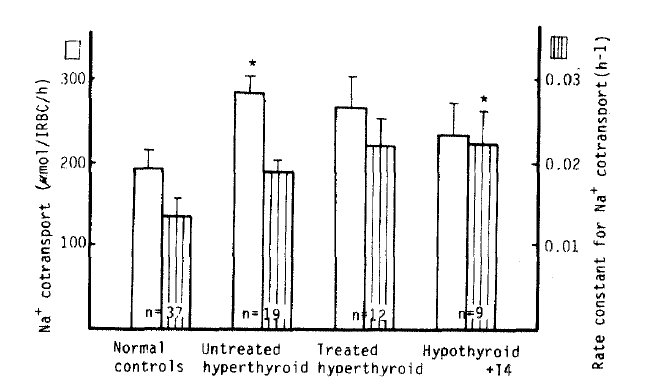
Fig.┬Ā6.
Na+-Li+ countertransport in normal controls and hyperthyroidism. *p<.01 vs control. Mean┬▒S.E.M.
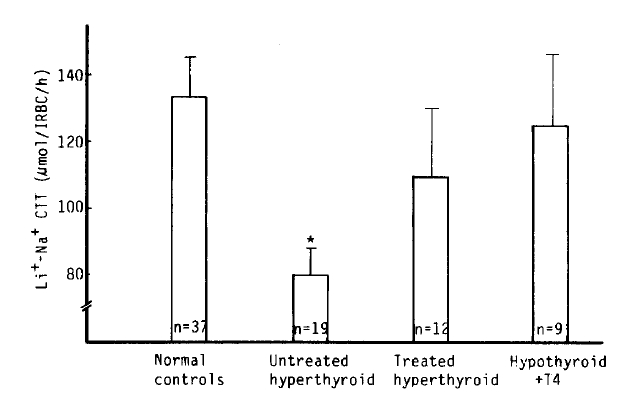
Fig.┬Ā7.
Passive Na+ permeability and rate constant tor passive Na+ permeability in normal controls and hyperthyroidism. *p<05 **p<.01 vs control. Mean┬▒S.E.M.
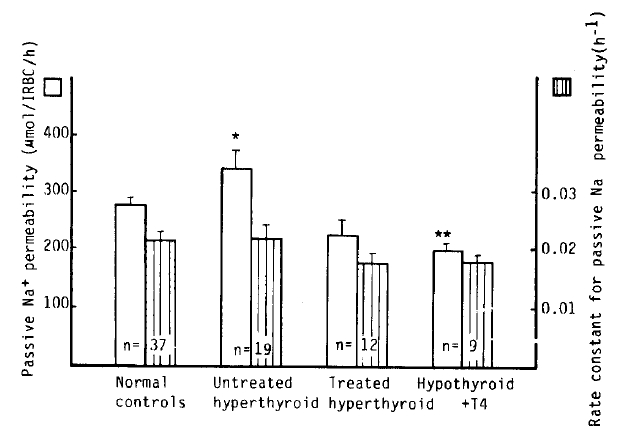
Table┬Ā1.
Patient Demographics and Thyroid Function in Each Group.
Table┬Ā2.
Intracellular Ionic Concentrations and Fluxes in Hyper- and Hypothyroid Subjects.
| Normal | Untreated Hyperthyroid | Treated Hyperthyroid | Hypothyroid + T4 | |
|---|---|---|---|---|
| [Na+]i (mmol/l RBC) | 13.6 ┬▒ 0.4 | 15.3 ┬▒ 0.7* | 12.1 ┬▒ 0.8## | 10.9 ┬▒ 0.7** |
| [K+]i (mmol/l RBC) | 105.8 ┬▒ 2.1 | 102.6 ┬▒ 1.7 | 99.6 ┬▒ 3.0 | 98.4 ┬▒ 3.0 |
| Na+-K+ pump (╬╝mol/l RBC/h) | 1,688 ┬▒ 52 | 2,083 ┬▒ 45**** | 1,837 ┬▒ 73## | 1,863 ┬▒ 93 |
| Rate constant for ouabain sensitive Na+ efflux (hŌĆō1) | 0.1359 ┬▒ 0.0059 | 0.1396 ┬▒ 0.0054 | 0.1568 ┬▒ 0.0086 | 0.1738 ┬▒ 0.0095** |
| Na+ CoT (╬╝mol/l RBC/h) | 192 ┬▒ 23 | 282 ┬▒ 20* | 266 ┬▒ 37 | 234 ┬▒ 39 |
| Rate constant for Na+ CoT(hŌĆō1) | 0.0134 ┬▒ 0.0022 | 0.0189 ┬▒ 0.0014 | 0.0221 ┬▒ 0.0031 | 0.0223 ┬▒ 0.0038* |
| K+ CoT (╬╝mol/l RBC/h) | 253 ┬▒ 28 | 225 ┬▒ 20 | 241 ┬▒ 38 | 258 ┬▒ 39 |
| Li+-Na+ CTT (╬╝mol/l RBC/h) | 133 ┬▒ 12 | 80 ┬▒ 8** | 109 ┬▒ 21 | 125 ┬▒ 21 |
| Passive Na+ permeability (╬╝mol/l RBC/h) | 276 ┬▒ 11 | 339 ┬▒ 33* | 222 ┬▒ 28 | 198 ┬▒ 13** |
| Rate constant for passive Na+ permeability (hŌĆō1) | 0.0214 ┬▒ 0.0014 | 0.0218 ┬▒ 0.0021 | 0.0178 ┬▒ 0.0015 | 0.0181 ┬▒ 0.0010 |
REFERENCES
1. Ismail-Beigi F, Edelman IS. Mechanism of thyroid catorinogenesis: Role of active sodium transport. Proc Nat Acad Sci 67:1071. 1970.



2. Ismail-Beigi F, Edelman IS. The mechanism of the calorigenic action of thyroid hormone. J Gen Physiol 57:710. 1971.



3. Ismail-Beigi F, Edelman IS. Effects of thyroid status on electrolyte distribution in rat tissues. Am J Physiol 225:1172. 1973.


4. Asano Y, Liberman UA, Edelman IS. Relationships between Na+-dependent respiration and Na+-K+ adenosine triphosphatase activity in rat skeletal muscle. J Clin Invest 57:368. 1976.



5. Folke M, Sestoft L. Thyroid calorigenesis in isolated, perfused rat liver; minor role of active sodium-potassium transport. J Physiol 269:407. 1977.



6. Curfman GD, Crowley TJ, Smith TW. Thyroid-induced alterations in myocardial sodium-and potassium-activated adenosine triphosphatase, monovalent cation active transport, and cardiac glycoside binding. J Clin Invest 59:586. 1977.



7. Biron R, Burger A, Chinet A, Clausen T, Dubois-Ferriere R. Thyroid hormones and the energetics of active sodium-potassium transport in mammalian skeletal muscle. J Physiol 297:47. 1979.



8. Lo CS, Angust TR, Liberman UA, Edelman IS. Dependent of renal (Na+-K+)-adenosine triphosphatase activity on thyroid status. J Biol Chem 251:7826. 1976.

9. Lin MH, Akera T. Increased (Na+-K+) ATPase concentrations in various tissues of rats caused by thyroid hormone treatment. J Biol Chem 253:723. 1978.


10. Philipson KD, Edelman IS. Thyroid hormone control of Na+-K+ATPase and K+-dependent phosphatase in rat heart. Am Physiol 232:C196. 1977.

11. Ismail-Beigi F, Edelman IS. Time course of the effect of thyroid hormone on respiration and Na+-K+-ATPase activity in rat liver. Proc Soc Exp Biol Med 146:983. 1974.


13. Smith TJ, Edelman IS. The role of sodium transport in thyroid thermogenesis. Fed Proc 38:2150. 1979.

14. Haber RS, Loeb JN. Early enhancement of passive potassium efflux from rat liver by thyroid hormone; relation to induction of Na, K-ATPase. Endocrinology 115:291. 1984.


15. Haber RS, Loeb JN. Effect of 3,5,3ŌĆÖ triiodothyronine treatment on potassium efflux from isolated rat diaphragm role of increased permeability in the thermogenic response. Endocriology 111:1217. 1982.

16. Cole CH, Waddell RW. Alteration in intracellular sodium concentration and ouabain-sensitive ATPase in erythrocytes from hyperthyroid patients. J Clin Endocrinol Metab 42:1056. 1976.


17. Garay RP, Nazaret C, Diez J, Etienne A, Bourgain R, Braquet P, Esanu A. Stimulation of K+ fluxes by diuretic drugs in human red cells. Biochem Pharm 33:2013. 1984.


18. Cuberbatch M, Morgan DB. Relations between sodium transport and sodium concentration in human erythrocytes in health and disease. Clin Sci 60:555. 1980.

20. Smith EKM, Samuel PD. Abnormalities in the sodium pump of erythrocytes from patients with hyperthyroidism. Clin Sci 38:49. 1970.


22. Dasmahapatra A, Cohen MP, Grossman SD, Lasker N. Erythrocyte sodium/potassium adenosine triphosphatase in thyroid disease and nonthyroidal illness. J Clin Endocrinol Metab 61:110. 1985.


23. DeLuise M, Flier JS. Status of the red cell Na, K-pump in hyper and hypothyroidism. Metabolism 32:25. 1983.


24. Lee MS, Oh Ys, Cho BY, Lee HK, Koh CS, Lee M. Change of Na-K ATPase activity of RBC membrance in hyperthyroidism. Kor J Int Med 27:1389. 1984.
25. Sterling K. The molecular mechanism of thyroid hormone action at the cellular level. The Thyroid Gland. In: Van Middlesworth L, ed. Chicago: Yearbook Medical Publishers INC, 203ŌĆō2291986.
-
METRICS

-
- 0 Crossref
- 1 Scopus
- 8,016 View
- 63 Download
- Related articles
-
Widespread intracranial calcifications in a patient with hypoparathyroidism2016 March;31(2)
Insulin Binding and its Action in Adipocytes of Hyperthyroid and Hypothyroid Rats1987 January;2(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


