INTRODUCTION
Gastric carcinomas arise from the mucus secreting cells of gastric crypts or from areas of intestinal metaplsia1).
Histologically, almost all gastric carcinomas are adenocarcinomas, but adenosquamous and squamous varieties comprise a very small portion (less than 1%)2).
Adenocarcinomas can be classified into four histologic types3,4): the diffuse type (synonymous with mucous or signet ring cell type), the intestinal type, the mixed type and the pylorocardiac gland cell type. Among the subtypes, pure signet ring cell type is very rare and has a poor prognosis2). In this type, numerous mucin vacuoles distend the cells and press the nucleus flat against the plasma membrane, resulting in the typical signet ring appearance.
Herein is presented a case of pure signet ring type early gastric carcinoma with extensive lymph node metastases.
REPORT OF A CASE
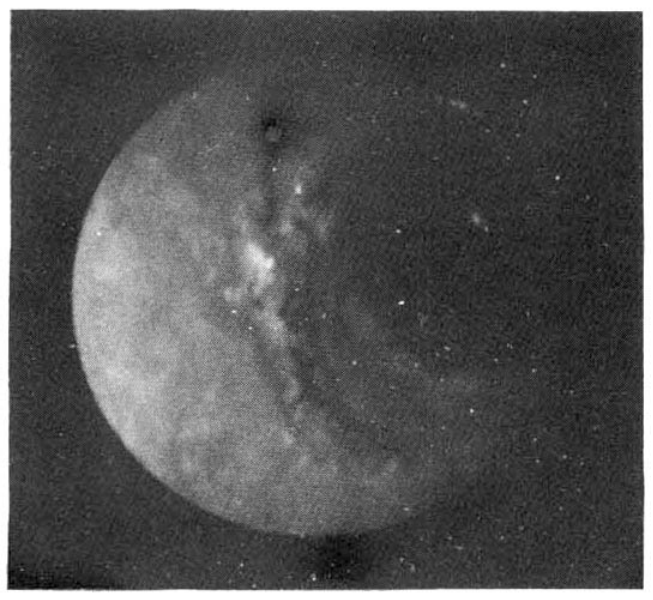
A 42-year old man was admitted to Kang Nam St. MaryŌĆÖs Hospital with indigestion and left upper quadrant pain. He was well until four months prior to admission, when he began to experience indigestion and left upper quadrant pain. An UGIS was performed, revealing a round 1.5 ├Ś 1.3 cm depression, suggestive of a chronic gastric ulcer or early gastric cancer (Fig. 1).
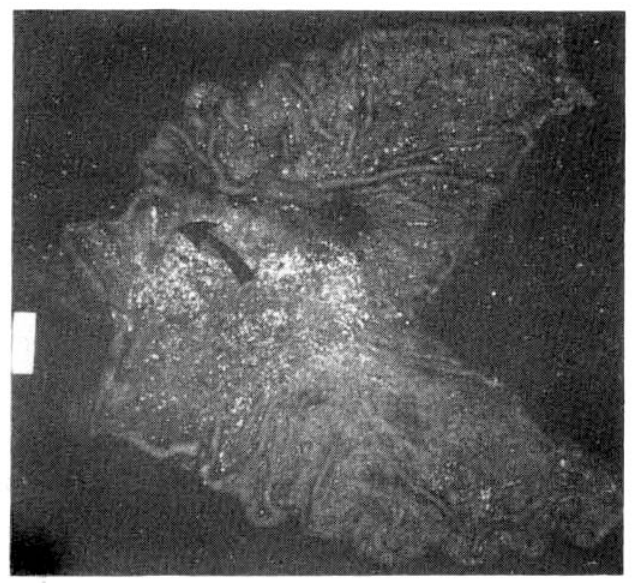
On gastrofiberscopic examination, a depressed, discolored lesion with converging irregular radiating folds in the antral portion like that seen in early gastric cancer (type IIc), was found and microscopic examination revealed signet ring cell carcinoma (Fig. 2,5,6).
The patient was admitted for further evaluation and treatment, on admission his vital signs were temperature 36.4┬░C, pulse 75, respiration 20 and blood pressure 110/80 mmHg.
On examination, the patient appeared to be slender and chronically ill. No lymphadenopathy was found. The head and neck were normal. The conjuctivae were not anemic and the sclerae were not icteric.
The lungs were clear and the heart was normal. In the left quadrant, rebound tenderness was absent. Liver and spleen were not palpable. Laboratory results included normal urinalysis, hemoglobin 14.8 g/dl; hematocrit, 43%; WBC, 5,800/mm3 with 67% neutrophils, 27% lymphocytes and 6% monocytes, and a platelet count of 204,000/mm3. Blood chemistries were nitrogen 14.7 mg/dl, creatinine 1. 4 mg/dl, total protein 6.4 g/dl, albumin 4.2 g/dl, globulin 2.2 g/dl, cholesterol 215 mg/dl, total bilirubin 0.6 mg/dl, direct bilirubin 0.2 mg/dl and indirect 0.4 mg/dl. The serum aspartate aminotransferase was 18 units and the serum alanine aminotransferase 9 units.
The CEA was 1.7 ng/dl by RIA. The chest X-ray was normal and a computerized tomographic scan of the abdomen showed a no evidence of metastatic lesions. On the 4th hospital day, the patient was transferred to the General Surgery Department.
Surgery was performed on the 7th hospital day, revealing a shallow depression in the anteior wall of the greater curvature side in the antrum and multiple perigastric lymph node enlargement. A Billroth type II subtotal gastrectomy was performed and lymph nodes along the portal hepatis, celiac axis branch were removed.
Grossly, the subtotal stomach, 25 cm along the greater curvature and 11 cm along the lesser curvature, presented a pinkish brown, smooth serosal surface. An irregular, superficial depressed lesion, 3├Ś1.5 cm, was noted on the anterior wall of the greater curvature in the antrum, 3 cm from the distal pyloric ring, giving it a reddened and granular appearance with a relatively well defined margin. Small areas of the margins were slightly indurated and sloped toward the depression.
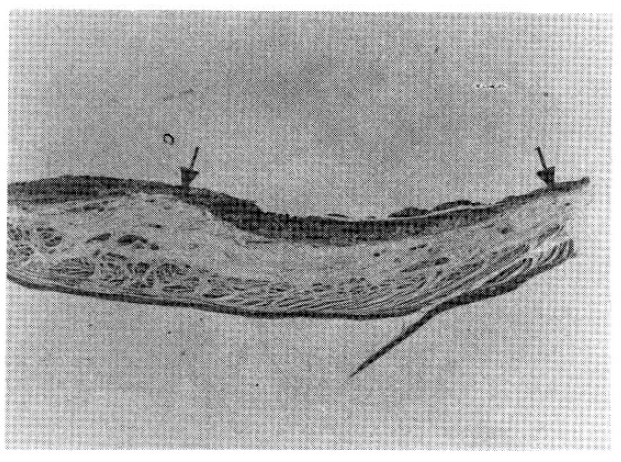
Microscopically, a pure signet ring cell carcinoma, confined by the muscularis mucosa but invading the lamina propria was observed. Areas of the carcinoma were infiltrated by isolated signet ring cells while others were composed of compactly aggregated signet ring cells. Lymph node involvement (metastases to 7 of 11 superior gastric nodes and to 10 of 14 inferior gastric nodes) was extensive, though the carcinoma was confined to the mucosa (Fig. 7).
On the 14th postoperative day, chemotherapy (5-FU, Adriamycin and Mitomycin) was administered to the patient and on the twenty third Hospital day, he was discharged in an improved condition.
On the 45th postoperative day, the patient received a second dose of KUR chemotherapy and was followed in the out-patient clinic.
DISCUSSION
Early gastric cancer has been defined as a carcinoma which is confined to the mucosa or submucosa, regardless of the presence of lymph node metastasis5).
In this case, a pure signet ring cell carcinoma located in the anterior wall of the greater curvature side in the antrum and restricted to the mucosa with no invasion of the muscularis mucosa was observed. Also widespread lymph node metastases were seen.
Histologically, diffuse type adenocarcinoma accounts for 33 to 40% of the cases of gastric carcinoma, the intestinal type varies from 22 to 53 % and pylorocardiac gland cell type, 28%3,4), but in Korea, Shim et al6)., reported in a review of 108 cases of gastric adenocarcinoma, that mucous cell carcinoma accounted for 62% of the cases; pylorocardiac gland cell carcinoma, 23%; intestinal cell carcinoma, 8% and unclassified type, 7%. In a review of 574 gastric carcinomas, Brander, et al1). classified mucous carcinomas into three main types: pure signet ring cell type, well differentiated type and mixed type.
Recently, the incidence of signet ring cell in gastric carcinoma biopsy and resection has risen sharply8).
The relationship between histologic types of gastric carcinoma and prognosis was investigated. Mulligan and Rember7) reported that mucous cell carcinomas composed of signet ring cells are more frequent in females than in males, occur at a comparatively younger age (less than 40 years old) and have a poor prognosis, while intestinal cell type and pylorocardiac gland cell type occur moe frequently after the age of 40 years and have a better prognosis than mucous cell type. Stemmermann and Brown9) pointed out that the 5 year survival rates were about 27.4% for intestinal type and 9.9% for diffuse type, but according to the Miwa report10), only the ŌĆśearlyŌĆÖ diffuse type had a better prognosis than ŌĆśearlyŌĆÖ intestinal type (5 year survival rates of 90% and 85% respectively).
The majority of the diffuse type shows mixed patterns, in which extracellular mucus and gland formation are present with signet ring cells, while there are only a few cases of pure signet ring cell type. The survival for pure signet ring cell type and mixed type is about 5 and 21 months respectively1).
In a study of 167 early gastric carcinomas, Kodama, et al5). reported that in the so-called ŌĆśsmall mucosal typeŌĆÖ a lesion of 4 cm. or less with only mucosal invasion, only one case exhibited lymph node metastasis.
Therefore, this case with widespread lymph node metastases although confined to the mucosa, is thought to be rare and highly malignant, although the pathogenesis is not yet known.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





