INTRODUCTION
MATERIALS AND METHODS
1. Collection of Tumor Specimens
2. Preparation of Tumor Single Cell Suspension
3. Culture Assay for Tumor Colony-Forming Cells
4. In Vitro Chemosensitivity and in Vivo Correlation
5. Morphologic Studies of Tumor Colonies
RESULTS
The overall growth rate was 71.4% (15 of 21). The growth rates of tumor cells obtained from ascites, pleural effusions and tumor nodules were 70.5% (12 of 17), 100% (2) and 50% (1 of 2) respectively (Table 1, 2).
The number of the colonies ranged from 5 to 96 per petri dish, and the median was 20. The PE ranged from 0.001 to 0.036%, and the median was 0.003% (Table 1, 2).
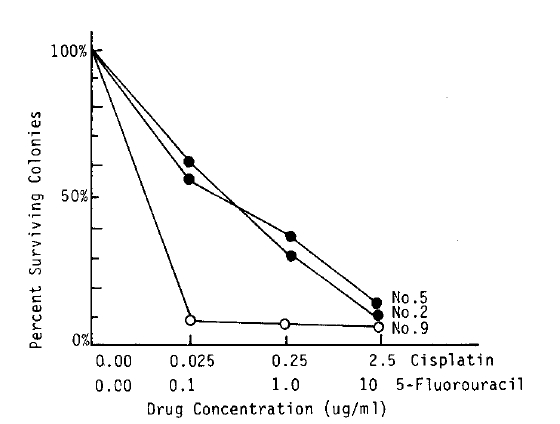
The in vitro chemosensitivity tests were performed in 5 patients, and the result showed 4 sensitive and 1 resistant to cisplatin (Table 3). In 3 cases sensitive to cisplatin, dose-related responses were seen (Fig. 1). The result of trial with intraperitoneal instillation of cisplatin revealed that 3 patients in vitro sensitive were responsive in vivo, and 1 patient in vitro resistant was non-responsive in vivo.
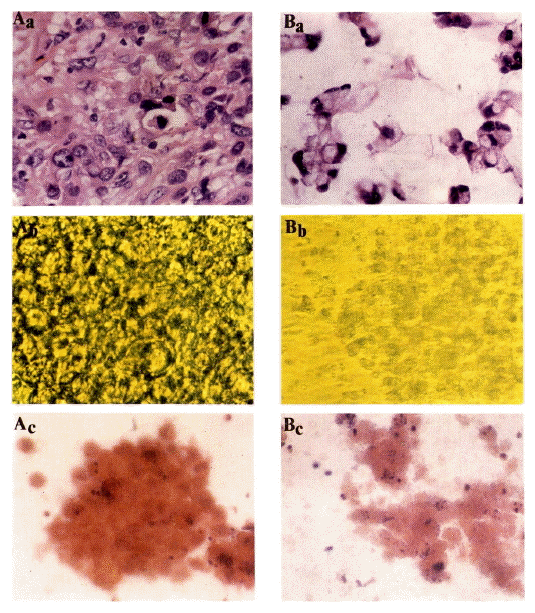
In morphologic studies, the colonies made of tightly packed cells were grown from the pooly differentiated adenocarcinoma whereas the colonies made of loosely packed cells with mucin formation were grown from the mucin secreting adenocarcinoma (Fig. 2).



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





