INTRODUCTION
The polymerase chain reaction (PCR) is currently the most sensitive technique for the detection of serum HBV DNA and is even more sensitive than chimpanzee infectious dose 50.1–5) With loss of HBeAg and seroconversion to anti-HBe in patients with chronic hepatitis B, serum HBV DNA may become undetectable by PCR,5,6) and a negative HBV DNA test in the serum by PCR has been implicated to be an indicator of a sustained remission.5,7,8) However, a few cases of reactivated chronic hepatitis B in patients without detectable serum HBV DNA by PCR has recently been reported in retrospective studies.5,9) Nevertheless, it has not been defined yet how frequently and when such reactivations occur after seroconversion to anti-HBe.
To evaluate the prognostic significance of the disappearance of serum HBV DNA by PCR after spontaneous HBeAg/anti-HBe seroconversion and concurrent or subsequent biochemical remission, we prospectively investigated the reactivation rates in chronic hepatitis B patients according to the positive or negative serum HBV DNA test by PCR.
MATERIALS AND METHODS
1. Patients
We enrolled 28 patients with chronic hepatitis B (24 men and 4 women: mean age 34.1 yr) who spontaneously seroconverted to anti-HBe with concurrent or subsequent normalization of biochemical liver function tests. All of the patients had been followed up for more than a year before entry. The investigation was approved by our institutional Review Committee. HBV serological markers (HBsAg, HBeAg and anti-HBe) were determined by radioimmunoassay kits (AUSRIA-II, Abbott-HBe, Abbott Laboratories, North Chicago, III., USA), and biochemical liver function tests were analyzed by sequential multiple autoanalyzer. The mean period of initial serum collection was 4.4 months (2–8 months) after normalization of alanine aminotransferase (ALT). Serum HBV DNA was analyzed by PCR-Southern blot hybridization,10,11) and then the patients were divided into two groups according to the presence or absence of serum HBV DNA. The initial clinical and biochemical characteristics of the two groups are shown in Table 1. There were no statistical differences in baseline characteristics between the two groups. They have been prospectively followed up for 20–66 months (mean 55.5 months) with biochemical liver function tests every 1–3 months. The reactivation was defined as an abrupt elevation of ALT levels to beyond 2.5 times the upper normal limit.1)
2. Detection of Serum HBV DNA by PCR
A pair of primers from a conserved region of the S gene11) was used to amplify HBV DNA by PCR using a commercially available reagent kit (Gene Amp DNA Amplification kit, Perkin-Elmer Cetus, Norwalk, CT, USA) according to the manufacturer’s instructions. Amplified HBV DNA products were electrophoresed in a 3% NuSieve GTC Agarose gel (FMC Co., Rockland, ME, USA), transferred to Zeta Probe nylon membrane (BIO-RAD Laboratories, Richmond, CA, USA), and were hybridized with a 32P-labeled whole HBV-genomic DNA (ATCC No.45020, Rockville, MA, USA).10,11) Results were considered valid only if they were consistent in two independent experiments. The nucleotide sequence of pre-core region of HBV at the time of reactivation was analyzed by direct sequencing of PCR products as previously reported.12,13)
RESULTS
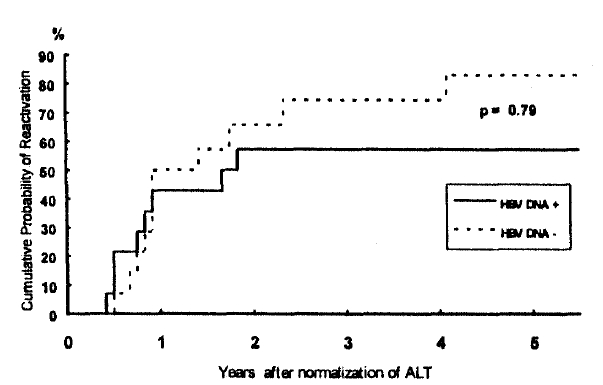
The cumulative reactivation rates among total patients were 46.4%, 61.3%, 65.6%, 65.6% and 70.5% at the end of 1st, 2nd, 3rd, 4th and 5th year, respectively; thus, the annual increments in reactivation rates were 14.9%, 4.3%, 0% and 4.9% by the end of 2nd, 3rd, 4th and 5th year after normalization of ALT, respectively. Therefore, most, if not all, episodes of reactivation (17/19) occurred within 2 years after normalization of ALT. The cumulative reactivation rates in patients with serum HBV DNA were 43%, 57%, 57%, 57% and 57% at the end of 1st, 2nd, 3rd, 4th and 5th year, respectively, and those in patients without demonstrable HBV DNA were 50%, 66%, 74%, 74% and 83%, respectively. The difference in the cumulative probabilities of reactivations between patients with and without serum HBV DNA was not statistically significant (p=0.79) (Fig. 1).
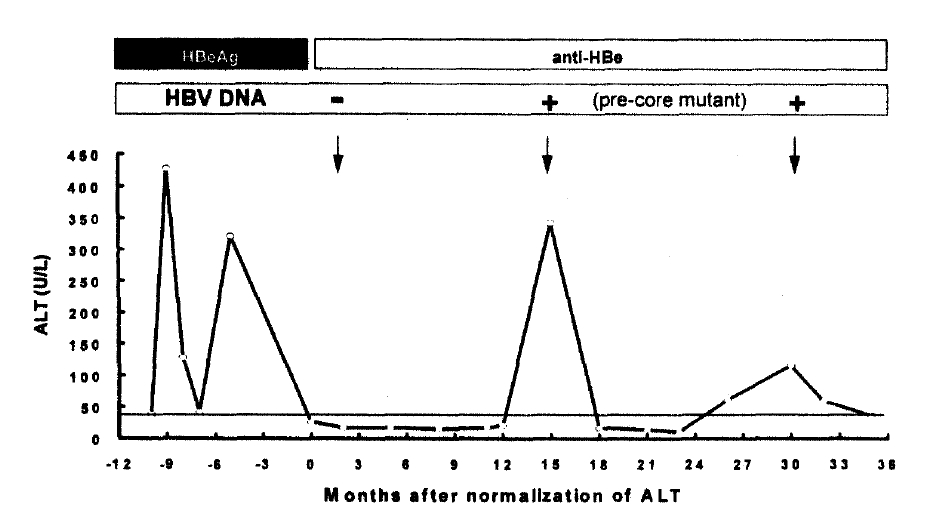
A representative case with undetectable serum HBV DNA, which subsequently experienced two episodes of reactivation, is depicted in Fig. 2. Both episodes were accompanied by the appearance of the pre-core mutant HBV with G to A mutation at nt 1896.
DISCUSSION
The detection rate (50%) of serum HBV DNA by PCR among patients with recent sero-conversion to anti-HBe in this study is lower than those (80–100%) of the previous studies.5,14–16) Since the prevalence of serum HBV DNA in patients with anti-HBe seroconversion may vary according to normal or abnormally elevated ALT levels, the lower detection rate in the present study may result from the exclusive enrollment of patients with normalized ALT levels.
In this prospective study, we demonstrated that a significant number of anti-HBe-positive patients, without detectable serum HBV DNA by PCR, were subject to experience the spontaneous reactivations as frequently as those with HBV DNA in the sera. One possible explanation for the frequent reactivations in patients with anti-HBe and undetectable serum HBV DNA is the persistent presence of low-level viral replication below the detection limit of PCR analysis. However, the highest sensitivity of PCR analysis, which could detect a single copy of HBV,2–4) does not support this possibility. A more probable one is the persistence of HBVs as a dormant state in the liver6) or other extrahepatic tissues such as peripheral-blood mononuclear cells17), which later resume active replication with the cessation of latent HBV infection.
Fattovich et al.18), who studied the progression of chronic hepatitis B by follow-up biopsies, showed the poor prognosis of even anti-HBe-positive and serum-HBV-DNA-negative (by dot blot) patients with chronic hepatitis B. However, they did not propose the reasonable explanation for the cause of progressive liver disease in these patients. Frequent reactivations observed in the present study even after seroconversion to anti-HBe, irrespective of serum HBV DNA status, may account for the poor prognosis observed by Fattovich.
The current definition of the response to α-interferon treatment has been the loss of serum HBV DNA analyzed by dot blot hybridization (not by PCR) along with biochemical normalization.1,19–21) However, PCR analyses give positive HBV DNA tests in a large portion (60–85%) of the responders to interferon therapy.1,5,22) Moreover, in some responders to interferon without detectable serum HBV DNA by PCR, HBV DNA may later reappear,1,22) suggesting that even the loss of serum HBV DNA by PCR after interferon treatment does not necessarily mean complete clearance of HBV particles. Thus, along with our results of frequent spontaneous reactivations in patients without serum HBV DNA by PCR, it is likely that the current definition of the response to interferon therapy might not be adequate.
The cumulative reactivation rate (46.4%) in total patients at the end of the first year in this study was much higher than the previously reported rates of about 10% per year in anti-HBe-positive patients.23,24) The difference may be due to the shorter intervals (1–3 months) between examinations with liver function tests in the present study than those of 3–6 month-interval in previous studies;23,24) therefore, the exacerbation episodes of short duration could have been missed in those studies. Another highly probable explanation is the different time point after seroconversion to anti-HBe at entry. In the previous studies,23,24) anti-HBe-positive patients were included, irrespective of the time point of preceding HBeAg/anti-HBe seroconversion, while, in the present study, only those patients who had seroconverted to anti-HBe shortly before the inclusion were investigated. This explanation is based on our present observation that most reactivations occur within 2 years after seroconversion to anti-HBe and subsequent normalization of ALT and thereafter, the mean annual reactivation rate is quite low (less than 10%). Our results, therefore, suggest that two years of maintained biochemical remission in patients with chronic hepatitis B, who spontaneously seroconverted to anti-HBe with normalization of ALT, is a better predictor of a further sustained remission than the determination of serum HBV DNA by PCR.
During reactivations of chronic hepatitis B in the anti-HBe-positive patients, serum HBeAg may reappear or not.12,13,25,26) In case of redeveloped HBeAg, the predominant causative agent was proven to be a wild-type HBV.12,25) Otherwise, it was predominantly the pre-core variant with a point mutation at nt 1896.12,13,26) The reactivation events of the present study were consistent with the latter finding



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





