 |
 |
| Korean J Intern Med > Volume 10(1); 1995 > Article |
|
Abstract
Objectives
Immune suppresion(IS) therapy has provided another opportunity of cure or improvement in the aplastic anemia patients who cannot receive bone marrow transplanatation due to many causes. There are a few reports regardig the factors that affect response, survial and prognosis after IS therapy, including antilymphocyte globulin(ALG) in aplastic anemia. Therefore, we analysed our experience to determine the prognostic factors.
Methods
Statistically analysed were 172 patients, from April 1982 to July 1992, who were diagnosed as severe aplastic anemia and treated with IS therapy, includig ALG, at Catholic University Medical College, St. Mary’s Hospital.
Results
Among 172 severe aplastic anemia(SAA)patients who entered the study from April 1982 to July 1992, 144 patients were analysed for response and 122 patient for survival. 58.4% (84/144)responded after the first course of IS therapy. Among those who did not respond on the first course an additional 44% (11/25) responded after the second course of IS therapy. Prognostic factors that might affect the response to the treatment and survival were analysed. In a univariate analysis of patients with no previous history of treatment before Is therapy, and a shorter interval between diagnosis and treatment, higher hemoglobin levels before IS therapy, and higher granulocyte counts and combined use use of cyclosporin A(CSA) were positively associated with response (p<0.05). The combined use of CSA during IS therapy, younger age, lower monthly requirement of platelets transfusion before IS therapy, higher leukocyte counts, higher percent of polymorphonuclear leukocytes, lower percent of lymphocytes, higer bone marrow cellularity and response were positively associated with survival(p<0.05). In a multivariate analvsis, shorter interval between diagnosis and treatment, no combined use of hemopoietic stimulants, such as androgen, and lesser total amount of transfusion were positively associated with Response (p<0.05). Higher leukocyte counts befors IS therapy and the combined use of CSA during IS therapy were significantly associated with longer survival(p<0.05). Patients with complete or partial response had excellent prognosis(96.7%–100% of 5 year survival rates). In contrast, patients with no response after IS therapy had 45.1% of 5 year survival rates.
Conclusions
With these results from the retrospective study of IS therapy, we find many valuable factors that have an influence on response or survival. IS therapy improves the survival of responded patients with SAA, and we confirmed that IS terapy is an important therapeutic tool for the SAA patients who are not feasible candidates for bone marrow transplantation.
Aplastic anemia is characterized by peripheral blood pancytopenia, variable bone marrow hypocellularity and the absence of underlying malignant or myelo-proliferative disease1).
The pathogenesis and treatment of severe aplastic anemia(SAA) is still debated. But, the experiences of bone marrow transplantation (BMT) and immune-suppression(IS) therapy, including antilymphocyte globulin(ALG), bone marrow hemopoietic cell cultuer and immunological test, suggested that quantitative or qualitative hemopoietic stem cell disorder, bone marrow micro-environmental and immunological disturbances are possible causes of aplastic anemia1,2).
Nowadays the common treatment for patients with SAA, who have an HLA compatible donor, is BMT. But, if patients are old or have no HLA compatible donor, IS therapy with ALG can be an important adjunctive therapy.
For SAA, various groups have reported promising results of IS therapy with ALG either with or without bone marrow infusion3,4). We also experienced the efficacy of ALG therapy. However, no systemic analyses were done to fine the prognostic factors for the survival of patients after ALG therapy and thus we report here the result of such anslysis with 172 patients.
ALG(Lymphoser Verna, Serum and Vaccine institute, Swiss) was given in intravenous dose of 40mg per kg of body weight(BW) per day on days 1 to 4. Methyl prednisolone(Solumedrol, Upjohn, USA) was added to prevent several allergy reactions induced by ALG therapy and tapered as fast as possible. From 1986, cyclosporin A(Sandimmune, Sandoz, Swiss, CSA)was given orally 5mg per kg per day from days 12 to 2 months, and the dosage was reduced to 4 mg per kg of BW per day duing next the 4 months. Afterwards, responders received 2.5 mg per kg per day for 1 year, but non-responders were discontinued. Androgen was given to 92 patients, 1.5 mg per kg of BW per day, and maintained and tapered according to the patients hemoglobin level for a year. Some patients, who had not responded during 6 months after the first course of IS therapy, received another course of IS therapy.
Response to treatment was defined as sustained improvemet in peripheral blood counts, by the decrease of, or no need for, transfusions and by the absence of infection. The quality of response was analyzed according to the following criteria5). (1) a complete response(CR) was indicated by a normal hemotocrit, granulocyte counts of≥1 × 109 per liter and a platelet count of≥100 × 109 per liter. (2) a partial response(PR) was indicated by granulocyte≥0.5 × 109 per liter, the absence of infections and of the need for transfusion and by sustained increases over pretreatment values of all hemopoiectic cell lines and (3) the non-response(NR) was indicated by the absence of change or death. Patients had a monthly follow up schedule. Responses were evaluated when the above responses were sustained for at least 6 months(Table 2).
Previously known or potential prognostic factors in the IS therapy were collected by means of a questionnaire. The items investigated were: age, sex, ABO blood group, etiology, treatment history with conventional methods before IS therapy, numbers of transfusions, monthly requirements of blood products within 48 hours before IS therapy, Hepatitis B viral markers, bone marrow cellularity, frequency of IS therapy, combined use of CSA, combined use of hemopoietic stimulating agents such as androgen during IS therapy, interval between IS therapy and response(Table 3). Response rates were evaluated with the chi-square test. Patient survival was analyzed by the Kaplan-Meier’s product-limit estimates6). Survival was compared with a log-rank test and/or Wilcoxon test. Multivariate logistic regression on response rate and Cox model on survival rate were performed with the SAS(SAS Institute, Cary, N.C.)and BMDP(BioMeDical Computer Program) package.
There were 102 males and 70 females. The ages ranged from 2 to 68 years(median 22 years). The etiology was idiopathic in 156 cases, drug induced in 3 cases, post-hepatitis in 9 cases, paroxysmal nocturnal hemoglobinuria in 1 case and pregnancy associated in 3 cases(Table 1).
144 patients were evaluable among 172 patients and all of them received more then one course of IS therapy. After a course of IS therapy, 58 patients(40.3%) had CR and 26 patients (18.1%) had PR. the others were NR. So the response rate was 58.4%(84/144). The 28 patients with non-response then received another course of IS therapy with the same schedule. 25 patients could be traced and an additional 11 patients responded(CR; 8 patients, PR; 3 patients). So, an overall 95 patients (65%) responded with first and second course of IS therapy.
Among responders, 82 patients responded within 6 months and 2 patients responded after 6 months. The median time of initial response time just after ALG infusion was 46 days(range 5–482 days).
Statistically significant and positively influencing factors in the response rate in IS therapy were as follows: no history of previous treatment(p<0.011), short intarval between diagnosis and IS therapy(p<0.05), higher hemoglobin level before tratment(p<0.05), the larger percentage of granulocyte(p<0.046), no combined use of hemopoietic stimulants, such as androgen (p<0.018), and combined use of cyclosporin A (p<0.025). The analyses were done with chisquare test as uniariate analysis. When these factors were reanalyzed by multivariate stepwise logistic regression, positively influencing factors were the short interval between diagnosis and treatment, no combined use of androgen and lesser total amount of transfusion before IS therapy(p<0.05).
One hundred twenty two patients(survival 106, death 16) who were treated and followed up for at least 6 months were analyzed by the Kaplan-Meier product limit estimates. The median follow up interval was 16 months(range 6–70 months). In complete response group, survival rate was 97% at 70 months. No death was observed during 42 months in partial response group. In non-response group, 86% of patients survived for one year, 76% survived for two years and 45% survived for 5 years(Fig. 1). The median survival time was about 46 months.
Most common side effects during the period of ALG infusion were thrombocytopenia(84.9%) and fever(55.2%). These side effects coule be controlled by acetaminophen, steroids and platelets transfusion. Other side effects such as hypertension(33.7%), hyperglycemia(9.3). allergic reactions such as serum sickness(8.7%), clinically documented infections (8.7%). elevated liver enzyme(5.2%) and anaphylactic shock(1.2%) were noted but these were resolved with supportive measurements. After infusion of ALG there was an activation of miliary tuberculosis and two cases of diabetes mellitus. Four patients died, two because of cerebral hemorrhage and two due to fulminating hepatitis(Table 4).
Kaplan-Meier product limit estimates of each prognostic factor were computed and compared by the log-rank test and/or Wilcoxon test. The prognostic factors such as combined use of CSA (p<0.0150, log-rank test), younger age(p<0.0351, Wilcoxon test), lesser monthly requirement of platelets transfusion(p<0.0305, log-rank test), larger leucocytes count before IS treatment(p<0.0001, log-rank test), larger granulocytes counts(p<0.0127, log-rank test) and good response(p<0.0001, log-rank) were significantly and positively associated with survival. Multivariate analysis with Cox model were performed with those variables that are found to be positively associated with survival in a univariate analysis, corrected reticulocyte percent before treatment, counts of platelets before treatment and interval between diagnosis and IS treatment. The results were that the survival rate was increasing, the combined use of CSA during IS therapy(p<0.003) and larger leucocytes counts (p<0.008).
Although the exact pathogenesis of aplastic anemia is still debated, decrease or functional disorders of hemopoietic stem cell, hemopoietic microenvironmental disturbance and suppression of hemopoietic function by immunological causes were suggested1,2).
In the aplastic anemia patients, autologous bone marrow recovery often occured after allogenic bone marrow transplant ation with cyclophosphamide conditioning3,4). In syngenic bone marrow transplant ation between identical twins, rejection was observed without cyclophosphamide conditioning8,9). From these results, it has been suggested that pathogenesis of aplastic anemia was related to immune system disorders.
Mathe et al10) reported that bone marrow recovered in half of patients after ALG and haploidentical bone marrow infusion for regulation of immune system in aplastic anemia. Bacigalupo et al11) reported that survival rates were increased by the high dose of corticosteroid treatment in aplastic anemia. With these results, abnormal immune system such as autoimmune disease was clinically related with pathogenesis of primary aplastic anemia. Through in vitro cell culture test and flow cytometric analysis, it has been known that activated suppressor T lymphocyte inhibit colony formation of several kinds of hemopoietic stem cells12). But, how primary hemopoietic stem cells in bone marrow are injured by the immune system is not known untilnow.
The mechanism of action of ALG in aplastic anemia is still debated. But some authors suggested that this agent promotes the helper T cell and suppressor T cell ratio by removing activated helper T cells that inhibit hemopoiesis12,13) and stimulation. of both IL-2 and hematopoietic production induce hemopoiesis14).
We reported that in evaluable 144 patients, after a course of IS therapy, the complete response was 40.3% and total response including partial response was 58.4%. It was a similar finding to that which has been reported as to hematologic improvement in 30%–65% of treated patients3,4,15).
As for the factors influencing the response, the patients who had no history of previous treatments, such as androgens, had 43.1% complete response(p<0.003), but only 15.3% patients who had been treated with androgen had complete response Also, if the interval between diagnosis and treatment is shorter, especially within 3 moths, the response rate is higher(p<0. 001). The following factors were positively associated with response; higher hemoglobin concentration before treatment(p<0.05), larger percent of polymorphonuclear leucocytes(p<0.046) and combined use of CSA during IS therapy(p< 0.038).
Multivariate stepwise logistic regression revealed that the shorter interval between diagnosis and treatment(p<0.001), the lesser total amounts of transfusions before treatment(p<0.039), a higher response rate and no combined use of androgen during IS therapy(p<0.019) were positively associated with response.
Although it is difficult to assess the full impact of the results of multivarate analysis, it was known that the patients did not mrakedly respond without a shorter interval between diagnosis and treatment. So the choice of treatment would be important in the early stage after diagnosis.
Specifically, androgen was not positively influenced during IS therapy. It was known that testosteron reduces complement sensitivity of precursor cells in aplastic anemia patients treated with ALG16), therefore there is a possibility that administration of androgen during IS therapy lowers the response rates by suppressing the action of IS therapy. But androgen affects maintaining the hemoglobin levels in bone marrow recovery group after IS therapy. Further follow-up studies should be done with respect to androge.
The median of interval between diagnosis and treatment was about 7 weeks and was similar with that 6–8 weeks of Doney et al4).
Eighty-four patients (97.7%) responded within 6 months, except 2 who responded positively after 6 months. Of the total 28 patients who had nonresponse after the first course, an additional 44% responded after the second course. So we thought it to be effective if the patients were trated with a second course of IS therapy when it was evaluated that the patients had no response within 6 months after IS therapy.
The most important side effect during IS therapy with ALG was sudden thrombocytopenia. To prevent thrombocytopenic complications, such as cerebral hemorrahge or other bleeding disorders, platelets should be supplied adequately and properly before IS therapy. Though two cases of anaphylaxtic shock were noted during IS therapy, these could be controlled by antihistamin, corticosteroids and fluid therapy. Neither patient died by ALG infusion during IS therapy.
There were 2 patients who were normal in early treatment but acquired diabetes mellitus belatedly over 6 months after IS therapy, these two having the worse peripheral blood findings. In 2 cases among the evaluable patients, the number of platelets maintained above 20,000/uL, and these died of cerebral hemorrhage. Two patients died of fulminating hepatitis immediately after IS therapy. The cause of the cerebral vascular accident cases was not clear, and those of fulminating hepatitis, tuberculous pleurisy, hypertension and diabetes mellitus were thought to be related to the use of corticosteroids.
During the follow-up, 94% of patients survived over 1 year, 2 year survival rate was 88%, 3 year survival rate was 85% and 5 year survival rate was 77%. Those rates were higher than that of EGBMT17) in which 1 year, 2 year and 4 year survival rate was each 62.7% 56%, and 52 %, respectively, after immunosuppression therapy in 170 severe aplastic anemia.
In response group, five year survival rate was close to 100%(96.7% in CR group, 100% in PR group), and in the noresponse group, 5 year survival rate was 45.1 %(Fig. 1). So we suggested that there was an outstanding difference among the survival rates according to the response after IS therapy.
As to the above findings, the causes of the higher response rate than the western results were not clear but there are some possiblities as follows: 1) The pathogenesis of aplastic anemia were different in different races such as related HLA antigen, 2) it was thought that maintaining therapy with CSA resulted in a positive effect15), but further systematic studies are needed to clarify these hypothesis.
The positively influenced factors in univariety analysis on survival rate were combined use of CSA(p<0.050) during IS therapy, younger age(p<0.0351), lesser monthly requirement of platelets transfusion before IS therapy(p<0.0244), larger leukocyte counts(p<0.0107), larger percent of granulocytes(p<0.0003), lower percent of lymphocytes(p<0.0001), higher bone marrow cellularity(p<0.0127) and response(p<0.0001). With these results, we thought that the survival rate was higher in that PMNs or platelets were produced and maintained partially before IS therapy.
Although some other reports showed that the age of the patient did not influence the survival rates4,17), survival rates were better in younger patients in our experience.
In multivariete analysis, higer leukocyte counts before IS therapy(p<0.0010), and combined use of CSA during IS therapy(p<0.027), had a positive influence on survival.
According to our findings, for a good response toward cure and to prolong the survival time with IS therapy, it is recommended that there is a combined use of CSA, without the use of androgens during IS therapy, and that there is early treatment during a period of decreasing monthly requirement of platelets transfusion after diagnosis.
The result that CSA had a good influence on response and survival rate could be interpreted as follows. Cyclosporin is a noncytotoxic immune-suppressant which blocks interleukin-2 secretion and suppresses the induction of activated T cells and r-interferon, which suppress the CFU-GM in vitro13,18). It is important to maintain the state of IS, and the cycle of early immune reconstitution which might occurr shortly after the IS therapy is inhibited, but further studies should follow to know the detailed mechanism.
The factor excluded in this study was infection. But, this factor was excluded because the patients were not treated with IS therapy during an active infection state.
We suggest that IS therapy with ALG might be a useful therapeutic modality for patients whose bone marrow is not qualified due to old age or no HLA matched donor
Fig. 1.
Kaplan-Meier product limit estimates of survival according to the response of the aplastic anemia patients who got IS therapy.(CR; complete response, PR; partial response, NR; none response, p<0.0001, Log-rank test)
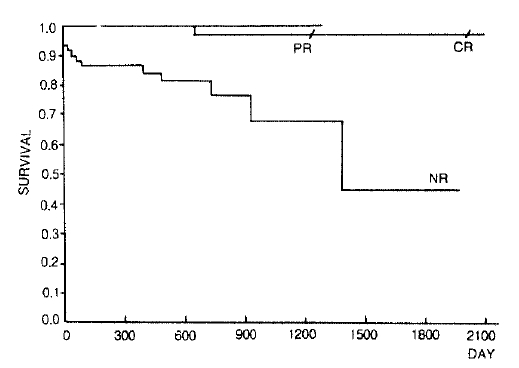
Table 1.
Profiles of Patients with Aplastic Anemia Who Received Immune Suppression(IS) Therapy with ALG
Table 2.
Clinical Response Criteria to Immune Suppression Therapy
Table 3.
Factors Considered in the Statistical Analysis of Immune Suppression(IS) Therapy
Table 4.
Side Effects and Death in Immune Suppression (IS) Therapy
REFERENCES
1. Gale RP, Champlin RE, Feig SA, Fitchen JH. UCLA conference, Aplastic anemia: Biology and treatment. Ann Int Med 95:477. 1981.


2. Camitta BM, Storb R, Thomas ED. Aplastic anemia (first of two parts); pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med 306:645. 1982.


3. Speck B, Conu P, Jeannet M, Nissen C, Burri HP, Groff P, Nagel CA, Buckner CD. Autologous marrow recovery following allogeneic marrow transplantation in a patient with severe aplastic anemia. Exp Hematol 4:131. 1976.

4. Doney K, Dahlberg SJ, Monroe D, Storb R, Buckner CD, Thomas ED. Therapy of severe aplastic anemia with antihuman thymocyte globuline and androgens: The effect of HLA-haploidentical marrow infusion. Blood 63:342. 1984.


5. Champlin RE, Ho W, Gale RP. Antilymphocyte globulin treatment in patient with aplastic anemia: A prospective randomized trial. N Engl J Med 308:113. 1983.


6. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Ass 53:457. 1978.

7. Thomas ED, Storb R, Gilbert ER, Longpre B, Weiden PI, Fefer A, Witherspoon R, Clift RA, Buckner CD. Recovery from aplastic anemia following attempted marrow transplantation. Exp Hematol 4:97. 1976.

8. Appelbaum FR, Fefer A, Cheever MA, Sanders JE, Singer JW, Adamson JW, Mickelson EM, Hansen JA, Greenberg PD, Thomas ED. Treatment of aplastic anemia by bone marrow transplantation in identical twins. Blood 55:1033. 1980.


9. Lu DP. Syngeneic bone marrow transplantation for treatment of aplastic anemia: Report of a case and review of the literature. Exp Hematol 9:257. 1981.

10. Mathe G, Amiel JL, Schwarzenberg L, Choay J, Trolard P, Schiveimer M, Hayat M, Schlumberg JR, Jasmin C. Bone marrow graft in a man after conditioning by antilymphocyte serum. Br Med J ii:131. 1970.



11. Bacigalupo A, Vanlint MT, Cerri R, Giordano D, Santini G, Carella M, Damasio E, Rossi E, Risso M, Vimercati R, Podesta M, Durando A, Reali G, Avanzi G, Barbanti M, Marmont AM. Treatment of severe aplastic anemia with bolus 6 methyl prednisolon and antilymphocyte globulin. Blut 41:168. 1980.
12. Zoumbos NC, Gascon P, Djeu JY, Trost SR, Young NS. Circulating activated suppressor T lymphocytes in aplastic anemia. N Engl J Med 312:257. 1980.

13. Kuriyama K, Tomonaga M, Jinnai I, Matsuo T, Yoshida Y, Amenomori Y, Yamadoa Y, Ichmaru M. Reduced helper(OKT4+); Suppressor(OKT8+) T ratio in aplastic anemia: Relation to immunosuppressive therapy. Br J Haematol 57:329. 1984.


14. Gascon P, Zoumbos NC, Scala G, Djeu JY, Moore JG, Young NS. Lymphokine abnormalities in aplastic anemia; Implications for the mechanism of action of antilymphocyte globulin. Blood 65:407. 1985.


15. Frickhoffen N, Kaltwasser JP, Schrezenmeier H, Raghavachar A, Vogt HG, Hermann H, Freund M, Meusers P, Salama A, Heimpel H, German Aplastic Anemia Study Group. Treatment of aplastic anemia with antilymphocyte globulin and methyl-prednisolone with or without cyclosporine. N Engl J Med 324:1297. 1991.


16. Nissen C, Deplanque MM, Wursch A, Moser Y, Carbonare VD, Speck B. Testosteron reduces complement sensitivity of precursor cells in aplastic anemia patients treated with antilymphocyte globulin. Br J Haematol 69:405. 1988.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



