 |
 |
| Korean J Intern Med > Volume 19(4); 2004 > Article |
|
Abstract
Protein-losing enteropathy is the manifestation of a diverse set of disorders, and it is characterized by the excessive loss of plasma proteins into the affected portions of the gastrointestinal tract, and this results in hypoalbuminemia. We report here on a case of severe protein-losing enteropathy with the typical clinical features of hypoalbuminemia, dependent edema and increased alpha 1-antitrypsin (╬▒1-AT) clearance, as measured by using 24hr stool testing. The associated disorder with the protein-losing enteropathy of our case was radiation enterocolitis and lymphatic obstruction that was due to radiation treatment and lymph node dissection in the remote past for the treatment of uterine cervical carcinoma. Our case suggests that chronic radiation enterocolitis can result in irreversible injury to the intestinal mucosa and a protein-losing enteropathy, which can bring about a very poor quality of life and even the loss of life.
Protein-losing enteropathy is not a disease, but rather it is the manifestation of a diverse group of disorders characterized by the excessive loss of plasma proteins into the lumen of the gastrointestinal tract, and this results in hypoproteinemia. The pathophysiology of protein-losing enteropathy can be summarized as increased mucosal permeability, mucosal erosions/ulcerations, and lymphatic obstruction1). There have been long list of diseases associated with protein-losing enteropathy2). We experienced such a case of severe protein-losing enteropathy as the late complication of pelvic irradiation for the treatment of uterine cervical carcinoma. Radiation-induced enterocolitis can manifest as a distressing protein-losing enteropathy, and it can result in a dreadful outcome.
A 66-year-old woman was referred to us by the gynecology clinic because of her progressively aggravated pitting edema of the bilateral lower extremities; in addition, she suffered from intermittent abdominal pain, persistent anemia with intermittent hematuria and hematochezia, and all of these symptoms had occurred during the previous 4 years.
26 years ago, she received a radical hysterectomy with pelvic lymphadenectomy and external irradiation at a dose of 6100 cGy for the treatment of her uterine cervical carcinoma (stage IB). About 23 years ago, she began to suffer from intermittent hematochezia and hematuria. She had received intermittent transfusions of packed RBCs, about a total of 8 to 10 pints yearly, under the presumptive diagnosis of radiation colitis and cystitis. 20 years ago, she experienced an episode of liver abscess that was treated by liver surgery. 7 years ago, gross hematuria had developed and she was diagnosed as having bladder cancer (stage I) with a background of radiation cystitis, for which she had undergone a transurethral bladder resection.
When we examined the patient, we observed severe anemia and a grade 3 pitting edema on the affected parts of her body, and cachexia was also noticed. Her abdomen was soft and mildly distended without any shifting dullness. Bowel sounds was moderately increased and there was neither tenderness nor rebound tenderness. The liver was not palpable and a previous operation wound scar was found on the right upper abdomen.
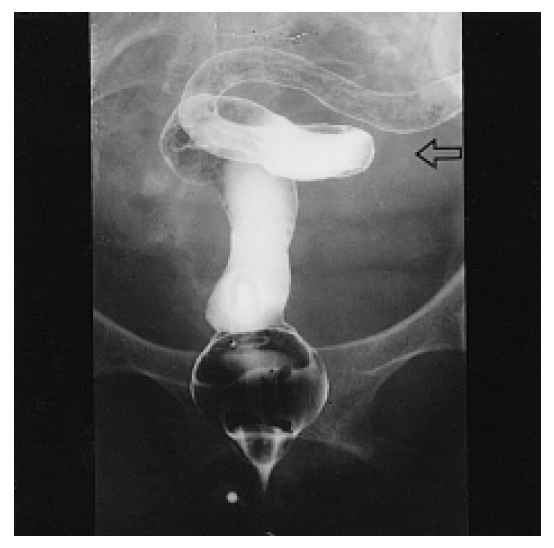
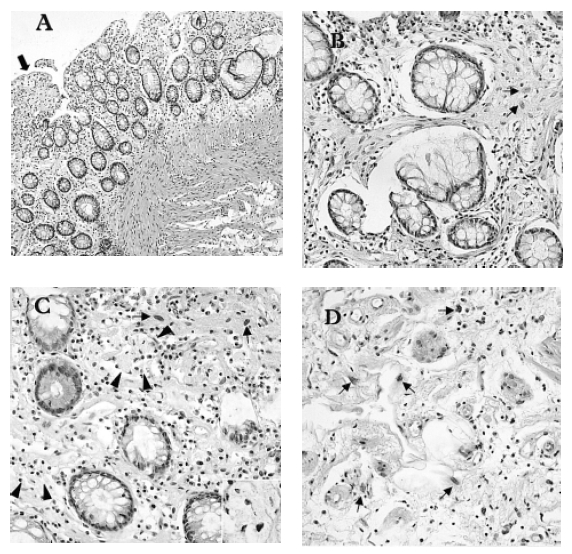
The laboratory tests revealed microcytic, hypochromic anemia (hemoglobin: 6.7 g/dL, normal: 12.0ŌĆō18.0 g/dL) with a serum iron level of 17 g/dL (normal: 37ŌĆō170 g/dL), a total iron binding capacity of 123 g/dL (normal: 250ŌĆō450 g/dL), and a ferritin level of 78 ng/mL (normal: 20.0ŌĆō300.0 ng/mL). The levels of vitamin B12 and folate in the serum were normal and the stool occult blood test was positive. The anemia was due to her chronic blood loss and chronic disease. The serum protein level was 4.7 g/dL (normal: 6.3ŌĆō8.2 g/dL), the albumin level was 1.6 g/dL (normal: 3.5ŌĆō5.1 g/dL), the cholesterol was 87 mg/dL (normal: 100ŌĆō200 mg/dL), and the serum total bilirubin, AST/ALT, alkaline phosphatase and prothrombin time were all normal. The BUN and creatinine levels were normal. The urinalysis results revealed microscopic hematuria (RBC 5ŌĆō9/HPF) and pyuria (>30/HPF) without proteinuria. Urine cytology didnŌĆÖt reveal any malignant cells, and the urine culture was negative. Stool examination was negative for fat and parasites. The result of the alpha-1- antitrypsin (╬▒1-AT) clearance test using 24hr stool collection was 169 mg/24hr (normal: 0.0ŌĆō27.0 mg/24hr), which was markedly increased and diagnostic for a protein-losing enteropathy. The small bowel series showed multifocal thumb-printing lesions in the ileum with delayed passage (Figure 1). On the barium enema, there was diffuse luminal narrowing with the loss of haustral markings in the rectum and sigmoid colon. (Figure 2). The sigmoidoscope findings were diffuse mucosal friability and vascular ectasia, which were gross and microscopic findings compatible with radiation colitis (Figure 3). Chest X-ray, electrocardiogram, echocardiogram, lung function tests and upper endoscopic findings were unremarkable. Abdominal CT scan showed liver cysts (size: 2├Ś2 cm and 0.8├Ś0.8 cm) in segments 4 and 6, respectively. Cystoscopic examination showed the mucosal thickening with edema and vascular ectasia, but there was no evidence of tumor recurrence. 99mTc-human serum albumin scintigraphy showed localized radioactivity in upper abdomen (Figure 4A) on the 24hr delay image, which meant there was a small amount of bowel leakage of radiolabeled albumin in the ascending colon. 99mTc-labeled dextran lymphoscintigraphy showed a decreased inguinal uptake in the left ileac lymph nodes with delayed migration and collateral circulations (Figure 4B), suggesting lymphatic obstruction in that area.
The patientŌĆÖs symptoms persisted and she required transfusion of 1 pints of packed RBCs due to the anemia, and she also received infusion of 25 grams of albumin in a monthŌĆÖs time to reduce her edema and dyspnea.
About 5 months later, she was re-admitted with symptoms of fever and severe dyspnea. The laboratory findings showed azotemia (BUN 89 mg/dL, serum creatinine 5.0 mg/dL), leukocytosis (14,500/mm3, neutrophils 89%), hematuria and pyuria. On urine culture, Escherichia coli and Morganella morganii were evident. Her hemoglobin level was 9.6 g/dL, and the serum protein (4.8 g/dL) and albumin (1.6 g/dL) levels were very low. Abdominal ultrasonography showed bilateral hydronephrosis. The patientŌĆÖs condition was severe; she had a urinary tract infection associated with urinary flow stasis that was probably due to a neurogenic cause because there was no mechanical cause for the obstruction on the cystoscopic examination. The patient received prompt administration of broad spectrum antibiotics and percutaneous nephrostomy on the right kidney. The patient condition then gradually improved with recovery of the serum creatinine to 1.9 mg/dL. However, a generalized seizure suddenly developed on the 18th day of admission. The magnetic resonance image (MRI) of the brain demonstrated diffuse brain atrophy and multiple small high signal intensity lesions in the bilateral hemispheres, and this was suspected of being small vessel disease or vasculitis. Fluorescent antinuclear antibody and anti-neutrophil cytoplasmic antibody testing were both negative. The level of complement 3, complement 4, fibrinogen and fibrinogen degradation product were within the normal limits. The seizure was not controlled with phenytoin and carbamazepin, and it became progressively aggravated in terms of frequency and duration. On the 32nd day of admission, she finally expired after a severe attack of seizure.
The diagnosis of protein-losing enteropathy can be made based on an increase in the alpha-1-antitrypsin (╬▒1-AT) clearance. However, the ╬▒1-AT clearance may be invalidated by intestinal bleeding, because this condition falsely elevates the ╬▒1-AT clearance (╬▒1-AT clearance rises 5.5 mL/day for each 10 mL of intestinal bleeding)1,2). Our patient suffered from chronic intestinal bleeding, so that her markedly increased ╬▒1-AT clearance (169 mL/day) might have been a false-positive result. We assumed that her bleeding rate might not exceed 20 mL/day, because she required about 2 pints of packed RBC for 2 months to maintain her hemoglobin level. In addition, she had hypoalbuminemia and hypoproteinemia without proteinuria. Therefore, her increased ╬▒1-AT clearance was diagnostic for the protein-losing enteropathy, along with the other clinical and laboratory results. We used 24hr stool collection instead of 72hr stool collection because of her poor compliance. Some studies have shown that the single-day stool collection provided as useful data as the does the 3-day stool collection did3ŌĆō5). 99mTc-human serum albumin scintigraphy is also a useful test for the diagnosis and monitoring of enteric protein loss, although it is less sensitive than the ╬▒1-AT clearance test6). Our patient showed diffuse, localized bowel activity in the right abdomen (colonic retention) on the 24hr delay scan, which was suggestive of bowel leakage, but the localization was not compatible with the radiological findings. 99mTc-labeled dextran lymphoscintigraphy of our case showed lymphatic obstruction at the left ileac lymph nodes with collateral circulations that was probably due to the previous lymph node dissection and pelvic irradiation, and this might well have contributed to the development of the protein-losing enteropathy.
Protein-losing enteropathy results from several pathologic alterations of the healthy mucosa. Mucosal injury causes increased permeability for the plasma proteins, and the mucosal erosions and ulcers result in the loss of an inflammatory, protein-rich exudate. Moreover, the lymphatic obstruction results in the dilatation of intestinal lymphatic channels and the rupture of lacteals that are rich in plasma proteins. The intestinal mucosa of our case displayed diffuse destruction from the ileum to the rectum with intermittent partial intestinal obstruction and chronic bleeding; these lesions were demonstrated by the barium studies and they were due to the previous irradiation. We thought the protein-losing enteropathy of our case was caused by mucosal injury and lymphatic obstruction that were the results of previous radiation and lymph node dissection.
The acute changes noted after radiation exposure are damages to the mucosal epithelial cells and the vascular endothelial cells. In the late phase after increasing doses of radiation, the ulceration, severe fibrosis and vascular ischemia progresses to stricture, fistulization and perforation7,8). Exposure to ionizing radiations has been shown to cause increased protein leakages in the gastrointestinal tract of laboratory animals. After an acute exposure of radiation, the intestinal protein loss developed within 24 hours and maximal loss was observed in 4th or 5th day after radiation. The mechanism of protein loss in this acute phase was an increased vascular permeability and an abnormality of cellular membranes in the intestine9). Metabolic studies with 131I albumin in the patients with bladder cancer or renal cell carcinoma showed an increased albumin loss across the intestinal tract after radiation therapy10). Case reports of radiation enterocolitis also have described hypoalbuminemia as a manifestation of these complications11).
The occurrence of radiation injury is dose dependent, and 90% of the patients having gastrointestinal complications have received more than 3,000 cGy. Our patient received a high dose of irradiation, 6,100 cGy, which resulted in severe, late complications. The development of chronic radiation enterocolitis is highly variable with a mean incidence of 5% to 15%7). The management of chronic radiation enterocolitis remains a challenge because of the progressive evolution of the lesions, which are eventually observed mainly as obstructive endarteritis and fibrosis9). The treatment should be as conservative as possible because of the diffuse nature of the lesions and the high morbidity associated with surgery12). Conservative therapy includes loperamide for diarrhea, antibiotics for bacterial overgrowth, nutritional support for malnutrition and endoscopic coagulation for the bleeding lesions. The use of corticosteroids or sulfasalazin is usually not consistently helpful because of the lack of inflammation in chronic radiation disease8,13).
Our case was a severe protein-losing enteropathy due to radiation enterocolitis and lymphatic obstruction that was the result of treatment for uterine cervical carcinoma in the remote past. Her clinical course showed nearly the whole spectrum of complication for pelvic irradiation such as enterocolitis that manifested as protein-losing enteropathy, continuous intestinal bleeding, partial obstruction of the intestine and also liver abscess that was probably related to the incompetent intestinal mucosal barrier; all of this was in addition to the hemorrhagic cystitis with secondary neoplasm of the bladder cancer, urinary stasis and severe urinary tract infection. Although she recovered from the uterine cervical carcinoma, her quality of life was miserable after the treatment for the cancer.
The treatment of radiation enterocolitis is very limited, so that prevention is the best way to avoid these problems. To reduce these complications, better radiation technique and better patient selection for the use of radiotherapy is definitely warranted. New therapeutic trials with treatment methods such as hyperbaric oxygen therapy and the search for the effective radioprotective agents14) should be continued.
Figure┬Ā1.
Small bowel series shows multifocal luminal narrowing with thumb-printing appearance of lesions (empty arrow) in the ileum.

Figure┬Ā2.
Barium enema shows diffuse luminal narrowing with the loss of haustral markings (empty arrow) in the rectum and sigmoid colon.
Figure┬Ā3.
Histological findings showing the delayed change of radiation- induced colitis (colonoscopic biopsy) (A) Low-power magnification shows mucosal fibrosis (black arrows) and thickening of muscularis mucosa with irregular arrangement of muscle fibers (H&E, ├Ś100). (B), (C) The mucosa shows increased collagen, atypical fibroblasts (small arrows) and collections of foam cells (arrow heads and inset) of the lamina propria, and atrophic glands with goblet cell depletion (H&E, ├Ś400). (D) High-power magnification of the submucosa reveals atypical cellular change of stromal fibroblasts (small arrows), increased and dilated capillaries with atypical endothelial cells, and the edematous stroma containing lymphocytes and eosinophils (H&E, ├Ś400).
Figure┬Ā4.
(A) 99mTc-human serum albumin scintigraphy shows localized radioactivity in the upper ascending colon on the delay image. (B) 99mTc-human dextran lymphoscintigraphy reveals the decreased uptake in the left ileac lymph nodes with delayed migration and collateral circulations, suggesting lymphatic obstruction.

REFERENCES
1. Kim KE. Protein-losing gastroenteropathy. In: Feldman M, Friedman LS, Sleigenger MH, eds. Sleigenger & FordtranŌĆÖs gastrointestinal and liver disease. 7th ed. 446ŌĆō452Philadelphia: WB Saunders, 2002.
2. Goldberg RI, Calleja GA. Protein-losing gastroenteropathy. In: Haubrich WS, Schaffner F, Berk J, eds. Bockus gastroenterology. 5th ed. 1072ŌĆō1086Philadelphia: WB Saunders, 1995.
3. Magazzu G, Jacono G, di Pasquale G, Sferlazzas C, Tedeschi A, Santoro S, Nibali SC, Musso F, Balsamo V. Reliability and usefulness of random fecal alpha 1-antitrypsin concentration: further simplification of the method. J Pediatr Gastroenterol Nutr 4:402ŌĆō4071985.


4. Hill RE, Hercz A, Corey ML, Gilday DL, Hamilton JR. Fecal clearance of alpha 1-antitrypsin: a reliable measure of enteric protein loss in children. J Pediatr 99:416ŌĆō4181981.


5. Quigley EM, Ross IN, Haeney MR, Holbrook IB, Marsh MN. Reassessment of feacal alpha-1-antitrypsin excretion for use as acreening test for intestinal protein loss. J Clin Pathol 40:61ŌĆō661987.



6. Chiu NT, Lee BF, Hwang SJ, Chang JM, Liu GC, Yu HS. Protein-losing enteropathy: diagnosis with 99mTc-labeled human serum albumin scintigraphy. Radiology 219:86ŌĆō902001.


7. Nguyen NP, Antoine JE. Radiation enteritis. In: Feldman M, Friedman LS, Sleigenger MH, eds. Sleigenger & FordtranŌĆÖs gastrointestinal and liver disease. 7th ed. 1994ŌĆō2004Philadelphia: WB Saunders, 2002.

8. Dubois A. Radiation injury to the gut. In: Haubrich WS, Schaffner F, Berk J, eds. Bockus gastroenterology. 5th ed. 1672ŌĆō1684Philadelphia: WB Saunders, 1995.
9. Vatistas S, Hornsey S. Radiation-induced protein loss into the gastrointestinal tract. Br J Radiol 39:547ŌĆō5501966.


10. Birke G, Jacobsson F, Liljedahl SO, Plantin LO, Wetterfors J. Catabolism of albumin and gamma globulin after treatment with ionising radiation to the abdomen. Acta Radiol Ther Phys Biol 6:113ŌĆō1211967.


11. Alp MH, Seymour AE, Grant AK. Radiation enterocolitis: a report of seven cases: some aspects of pathology and management. Aust N Z J Surg 40:144ŌĆō1511970.


12. Shiraishi M, Hiroyasu S, Ishimine T, Shimabuku M, Kusano T, Higashi M, Muto Y. Radiation enterocolitis: overview of the past 15 years. World J Surg 22:491ŌĆō4931998.


13. Kapadia CR. Inflammatory disorders other than ulcerative colitis. In: Spiro HM, ed. Clinical gastroenterology. 4th ed. 619ŌĆō630New York: McGraw-Hill, 1993.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



