INTRODUCTION
Helicobacter pylori has remarkably high urease activity1, 2), which provides a useful means of detecting its presence. The widely used methods of diagnosing a H. pylori infection based upon urease activity are the rapid urease test (RUT) requiring an endoscopy and a 13C or 14C urea breath test (UBT)3–6). These techniques take 1 to 24 hours and the final result of the RUT is usually not available until the next day at the earliest. Other investigators have tried measuring gastric juice urea and ammonia concentration as new diagnostic tests that may be performed at the time of an endoscopy and rapidly interpreted, so that patients can be treated for a H. pylori infection immediately after an endoscopic examination7, 8). They found that H. pylori infected patients have significantly lower urea levels, higher ammonia concentrations, and higher gastric urease activities than uninfected control patients. However, they noted that there is significant overlap in gastric juice ammonia concentrations between infected and uninfected patients. Therefore, the diagnostic role of gastric juice ammonia is still controversial. Kearney et al9). concluded that the measurement of a gastric juice ammonia concentration is a relatively insensitive and nonspecific means of diagnosis for H. pylori. Other Korean investigators compared gastric juice ammonia determination to a RUT and found that the gastric juice ammonia test has higher true-positive and lower false-positive rates than does the RUT10).
The effect of a H. pylori infection on acid secretion, and the relationship between gastric pH and a H. pylori infection and gastric mucosal histology have also been investigated11, 12). Furuta et al. found that a H. pylori infection and gastritis in the corpus suppress acid secretion and increase gastric pH, resulting in hypergastrinemia, and that the eradication of H. pylori normalizes acid secretion and serum gastrin levels12). However, the relationship of gastric juice pH and ammonia concentration with the parameters of a H. pylori infection was not investigated.
The pathophysiology of H. pylori gastritis remains incompletely understood. Previous investigators have hypothesized that the type and severity of inflammation in the gastric mucosa may be related to the amount of ammonia produced by H. pylori with urease activity13).
We determined the gastric juice pH and ammonia concentration in H. pylori gastritis, and looked for correlations between the two gastric juice parameters, and their relationship to H. pylori status and gastric mucosal histology, such as H. pylori density, gastritis severity, and gastritis activity.
MATERIALS AND METHODS
Subjects and study design
The subjects were 143 patients with dyspepsia scheduled for an elective endoscopy at the Gyeongsang National University Hospital, and who were eligible for inclusion in the study. After receiving informed consents, patients were interviewed, and data collection forms were completed, in which all clinical information was recorded. Patients were excluded from the study if they had taken antibiotics, proton pump inhibitors or bismuth compounds, or had undergone treatment for H. pylori during the preceding 4 weeks. Patients with renal insufficiency or liver cirrhosis were also excluded.
All subjects underwent an upper endoscopy and tests for H. pylori. Patients with endoscopically proven peptic ulcers or erosions with a H. pylori infection were submitted to ulcer treatment and H. pylori eradication. Patients received 20 mg of omeprazole or 10 mg of rabeprazole, 1,000 mg of amoxicillin, and 500 mg of clarithromycin twice daily for a week, and a proton pump inhibitor for an additional 5 or 7 weeks. The endoscopic examination and tests for H. pylori were performed at least 4 weeks after treatment.
The 143 patients enrolled in the study consisted of 74 men and 69 women, with a mean age of 49.9 ± 12.5 (range 19 to 71) years. The endoscopic diagnoses were gastric and/or duodenal ulcer (23 patients), erosive gastritis (12 patients), gastric dysplasia (10 patients), reflux esophagitis (5 patients), hyperplastic polyp (4 patients), submucosal tumor (1 patient), and chronic atrophic gastritis (88 patients). A H. pylori infection was documented in 94 patients (65.7%)(Table 1).
We compared gastric pH and ammonia concentration according to the status of the H. pylori infection, and examined correlations between the two biochemical parameters and their relationship to H. pylori density, gastritis severity, and gastritis activity.
Collection of samples
Immediately after the insertion of the endoscope into the stomach, 10–20 mL of gastric juice was aspirated through the suction channel of the endoscope and collected in a trap placed in the suction line. A routine inspection of the upper gastrointestinal tract was performed, and then at least three biopsy specimens from both the antrum and the corpus of the greater curvature were obtained for the rapid urease test and histology.
Determination of gastric juice pH and ammonia concentration
The pH and ammonia content of gastric juice were measured just after collection. Gastric juice pH was measured with a glass electrode pH meter. After centrifugation of the gastric juice samples at 3,000 rpm at 4°C for 10 min and dilution in 0.2 mol/L phosphate buffer at pH 7.4, the ammonia concentration in the supernatant was measured using an automated enzymatic method. An enzymatic kit (Synchron® system, Beckman Coulter Inc., Fullerton, CA, USA) specific for the determination of plasma ammonia concentrations was used. This method is based on the reaction between NH3, β-NADPH, H+ and α-ketoglutarate in the presence of the enzyme, glutamate dehydrogenase. The concentration of ammonia in the samples was determined from spectrophotometric readings at 340 nm.
Detection of H. pylori and histology of gastritis
All patients underwent a RUT and histologic examination, and/or 13C-UBT. Three antral and three corpus biopsy samples were obtained from all cases. One antral and one corpus biopsy samples were used for the RUT and the other samples were stained with hematoxylin-eosin. Histologic interpretation of gastric biopsies was performed by a pathologist who was an expert at identifying H. pylori. The grading of gastritis was performed according to the updated Sydney system14). The variables used for grading gastritis (H. pylori density, gastritis severity, gastritis activity) were assessed on a four-point scale ranging from 0–3: normal = 0; mild = 1; moderate = 2; severe = 3. H. pylori density was defined as the density of H. pylori colonizing the gastric mucosa, gastritis severity as the presence of chronic inflammatory cells (mononuclear cells) in the lamina propria, and gastritis activity as the presence of neutrophil polymorphs in a background of chronic inflammation. A 13C-UBT (HelikitTM, Isodiagnostika, Edmonton, Canada) was performed after the completion of the endoscopy, or on another day after an overnight fast. A baseline breath sample was collected into a collection tube. An aliquot of 75 mg of 13C-urea dissolved in 75 mL of citric acid solution was orally administered. Another breath sample was collected 30 minute after the ingestion of the urea. The breath samples were subsequently analyzed to determine the 13C/12C ratio by mass spectrometry (HeliView®, MediChems, Seoul, Korea). The UBT was considered positive if the difference of the 13C value over the baseline at 30 min was greater than 4‰. The H. pylori infection and eradication of the infection were judged on the basis of the RUT results, histologic examination, and 13C-UBT. H. pylori infection was defined as the case in which at least one of these tests was positive. H. pylori eradication was defined as the case in which all tests were negative.
Statistics
Data are expressed as medians (pH) or means standard deviation (ammonia). Differences in gastric juice pHs between H. pylori negative and positive groups, and between H. pylori eradicated and non-eradicated groups were analyzed by the Mann-Whitney U-test. Differences in ammonia concentrations between H. pylori negative and positive groups, and between H. pylori eradicated and non-eradicated groups were analyzed by the Student’s t-test. The Spearman’s correlation test was applied to the tests if the gastric juice pH was found to be associated with the ammonia concentration in gastric juice, and if the gastric juice pH or ammonia level was found to be related to H. pylori density, gastritis severity, or gastritis activity. Multivariate analysis of the variables influencing gastric juice pH was performed by multiple linear regression analysis. A p <0.05 was considered statistically significant. Statistical analyses were performed with SPSS 10.0.7 (SPSS Inc., Chicago, Illinois, USA).
RESULTS
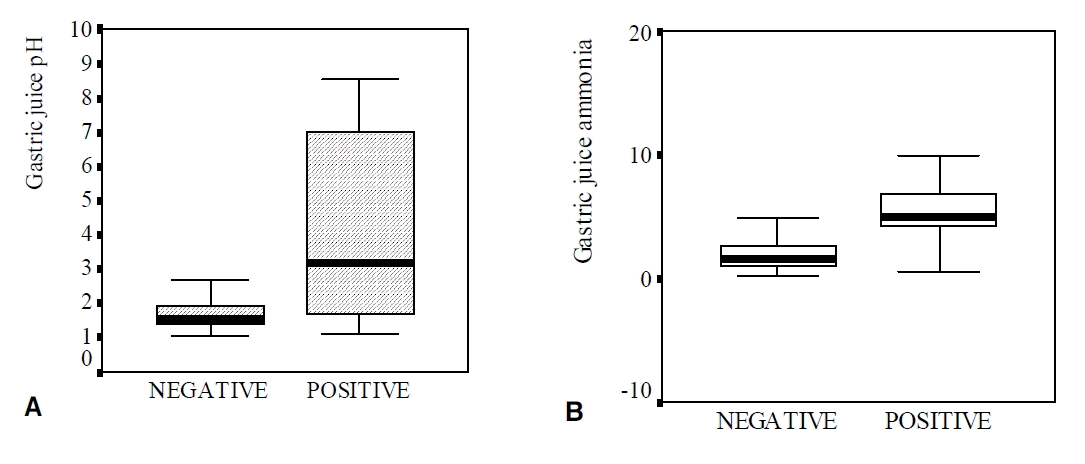
Ninety-four patients (65.7%) were infected with H. pylori and 49 (34.3%) were not infected (Table 1). Seventy-one patients had all three positive tests, 21 had two positive tests, and two had only one positive test. Gastric juice pH was significantly higher in the H. pylori-infected group than in the uninfected group (3.16 vs. 1.55, p=0.0001) (Table 2, Figure 1A). The ammonia concentration in gastric juice was significantly higher in the H. pylori-infected group (5.58 ± 2.69 μmol/L) than in the uninfected group (2.00 ± 1.49 μmol/L)(p=0.0001) (Table 2, Figure 1B).
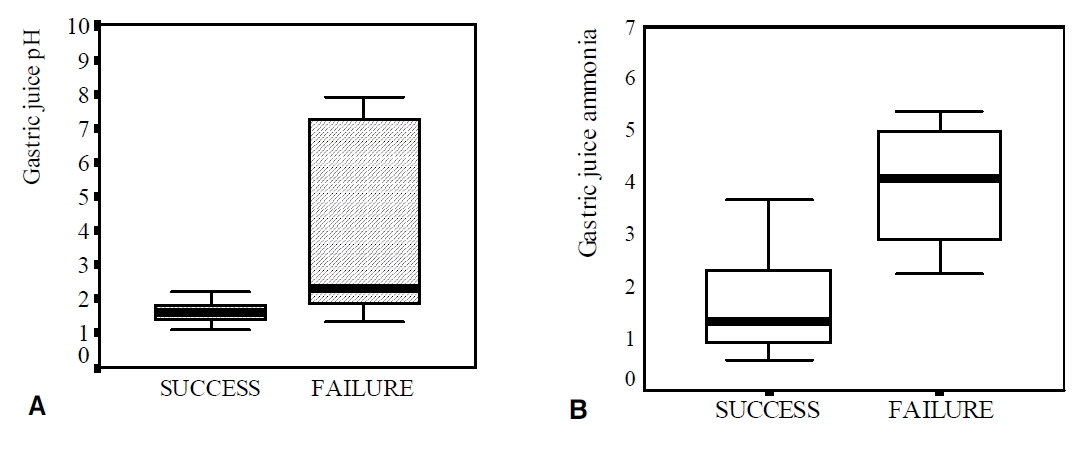
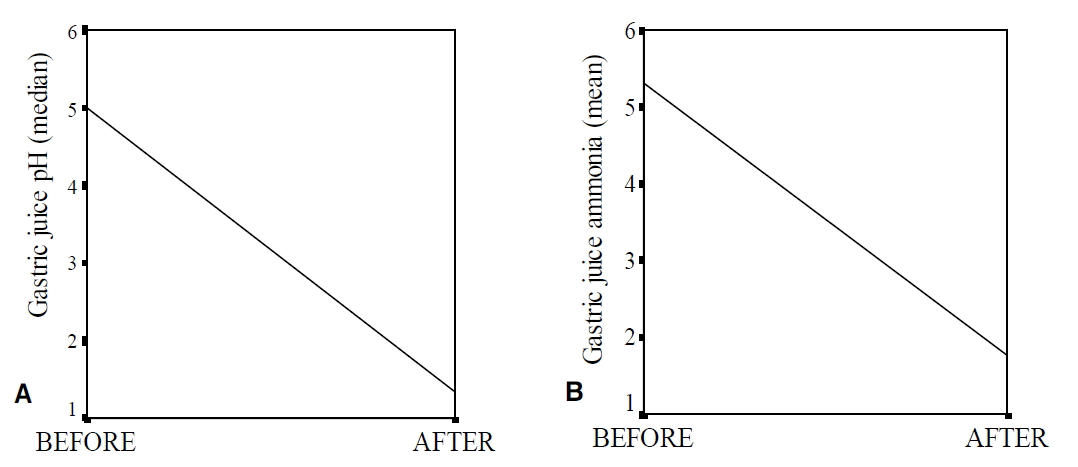
Among the patients with a peptic ulcer or erosive gastritis, 28 patients infected with H. pylori and who wanted eradication underwent eradication therapy. Treatment was successful in 19 patients (67.9%), and their gastric pH levels and ammonia concentrations were significantly lower than those of the nine patients who experienced eradication failure (1.60 vs. 2.33, p=0.007; 1.77 ± 1.28 vs. 4.02 ± 1.20 μmol/L, p=0.0001) (Table 3, Figure 2). The Gastric juice pH levels and ammonia concentrations were significantly decreased after the eradication of H. pylori, when compared to pre-eradication treatment levels in the 19 patients who experienced successful eradication (1.60 vs. 2.33, p=0.002; 1.77 ± 1.28 vs. 5.32 ± 2.58 μmol/L, p=0.047) (Table 4, Figure 3).
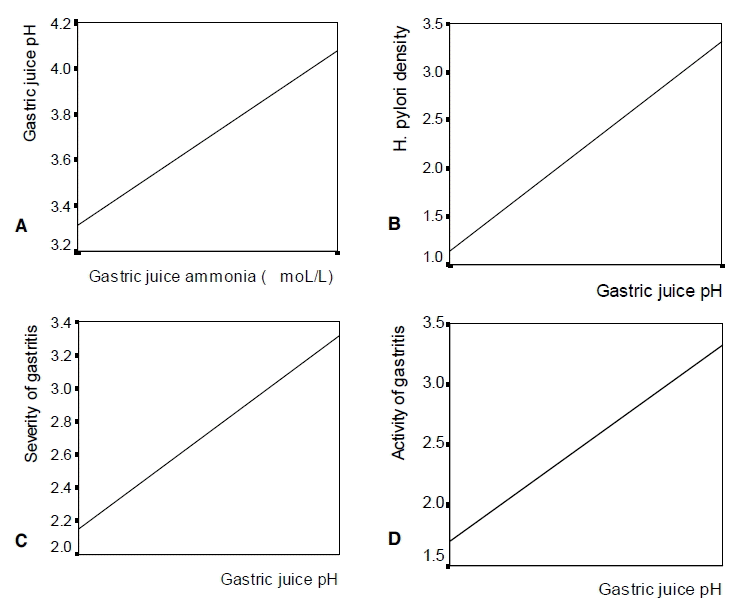
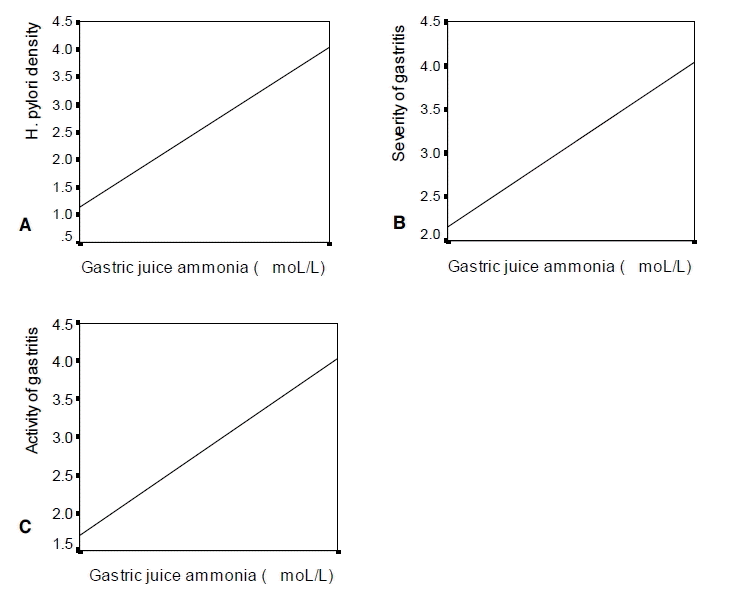
Gastric pH was significantly correlated with the intragastric ammonia level (rs=0.495, p<0.01), H. pylori density (rs=0.467, p<0.01), gastritis severity (rs=0.343, p<0.01), and gastritis activity (rs=0.418, p<0.01) (Figure 4). The gastric juice ammonia level was also significantly correlated with H. pylori density (rs=0.735, p<0.01), gastritis severity (rs=0.478, p<0.01), and gastritis activity (rs=0.579, p<0.01) (Figure 5). Multiple linear regression analysis was carried out for the variables such as age, sex, gastritis severity, gastritis activity, and gastric juice ammonia concentration. H. pylori density was excluded for analysis because the correlation coefficient between gastric juice ammonia concentration and H. pylori density was very high (multiple colinearity). Gastric juice pH was significantly associated with gastric juice ammonia concentration (p=0.025) and gastritis activity (p=0.018), but not with gastritis severity (p=0.386), sex (p=0.072), and age (p=0.366) (Table 5).
DISCUSSION
We found that H. pylori-infected patients had significantly higher gastric juice pH levels and ammonia concentrations as compared to uninfected subjects. In addition, high gastric juice pHs and ammonia concentrations were decreased after the eradication of H. pylori to significantly lower levels compared to uninfected groups. Other investigators have also found such encouraging results7, 8). However, they noted that there is a significant overlap in gastric juice ammonia concentrations between infected and uninfected patients7, 8), yielding a sensitivity for elevated gastric juice ammonia of 60%, specificity of 100%, positive predictive value of 100%, and a negative predictive value of 63% (using a cutoff value of 4.26 mM)7). Furthermore, findings in conflict with the previous encouraging results have recently been reported. Kearney et al.9) found that gastric juice ammonia concentration and the detection of ammonia using a rapid test device have inferior test characteristics as compared to previously published studies using currently available techniques. These findings suggest that the gastric ammonia level alone has limitations in the diagnosis of a H. pylori infection. Another study on the effect of a H. pylori infection on gastric juice pH observed that gastric juice pH is significantly higher in peptic ulcer patients (especially in gastric ulcer patients) than in control patients, and that gastric juice pH and serum gastrin levels in ulcer patients are significantly decreased compared to control levels after H. pylori eradication12). Most in vitro studies have also shown that H. pylori inhibits acid secretion. Several substances, including Nα methyl histamine produced by H. pylori15) and interleukin-1β (IL-1β) and tissue necrotic factor-α(TNF-α) induced by a H. pylori infection16, 17), are thought to inhibit acid secretion18, 19). In particular, IL-1β is known as a potent inhibitor of acid secretion; the peripheral and central administration of IL-1β potently inhibits gastric acid secretion in experimental animals19–23). IL-1β also directly inhibits acid secretion by cultured rabbit parietal cells, and impairs the function of enterochromaffin-like cells in vitro, which may be followed by a markedly decreased acid output24). Gastric acid secretion is decreased and serum gastrin levels are increased in Mongolian gerbils infected with H. pylori, and acid output and serum gastrin levels return to control levels after a recombinant human IL-1 receptor antagonist injection25). Cytokines and serum gastrin levels were not determined in our study, but our data on gastric juice pH agree with previous findings12). Furthermore, the median gastric juice pH in the H. pylori negative group in our study was 1.55, which is almost the same as in other studies12, 26). Previous studies and our findings suggest that gastric juice pH and ammonia concentrations are good indications of the status of a H. pylori infection, and they may be useful as compensatory tools in the diagnosis and eradication of a H. pylori infection.
We also analyzed the effect of gastric juice ammonia on gastric juice pH with sex, age, severity and the activity of gastritis by multiple regression analysis, and assessed the relationships between gastric pH and ammonia concentrations, between gastric pH and histology such as H. pylori density, severity and the activity of gastritis, and between gastric ammonia levels and histology. Gastric juice pH was significantly associated with gastric juice ammonia concentration (p=0.025) and gastritis activity (p=0.018). Furthermore, gastric juice pH and ammonia concentration correlated well with each other. Those levels also correlated well with H. pylori density, severity and the activity of gastritis. Bercik et al.27) have recently observed a higher gastric pH in H. pylori-positive subjects than in H. pylori-negative subjects during the administration of omeprazole, and concluded that a higher pH is predominantly attributable to neutralization by H. pylori-derived ammonia. Their conclusion agrees with ours. We did not performed a quantitative analysis of the relationship between ammonia production and gastric acidity, so we do not point to gastric ammonia as a major factor influencing gastric pH. However, according to our data, gastric juice ammonia produced by H. pylori appeared to contribute to the increase of gastric juice pH in active H. pylori gastritis. Urease of H. pylori protects the bacteria from gastric acid by the production of ammonia, which increases the pH in the microenvironment around the organism28). Direct injury to gastric epithelial cells occurs in vitro from ammonia28). Kearney et al.9) also reported on the relationship between gastric ammonia concentration and the severity of gastritis. It has been hypothesized that the ammonia produced by bacterial urease activity may play a role in the pathogenesis of gastritis13, 14). There are many evidences to support a role for ammonia in the pathogenesis of gastritis. There is a significant correlation between gastric juice ammonia concentration and the number of polymorphonuclear cells among patients with chronic renal failure with a high blood urea nitrogen concentration, upon gastric histological examination29). Oral administration of an ammonia solution to rats has also been shown to result in gastric mucosal injury30), and there is evidence to suggest that ammonia may impair the host immune response by decreasing the phagocytic activity of polymorphonuclear leukocytes in vivo31). Our study supports the notion that the severity and activity of gastritis may be correlated to an increased gastric ammonia concentration produced by a greater colonization of H. pylori, and gastric ammonia may play a partial role in the increased gastric pH in H. pylori gastritis.
Further studies are warranted to clarify the role of ammonia on gastric pH and the pathogenic role of ammonia in H. pylori gastritis.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





