INTRODUCTION
After its introduction to the area of anti-cancer therapy, thalidomide showed diverse activity toward several cancers1). Its most evident efficacy was reported in multiple myeloma2). Thalidomide shows an anti-myeloma effect through the anti-angiogenic activity, in addition to its selective cytokine inhibitory and immunomodulatory properties. However, the lack of a dose-response relationship and pharmacokinetic data, along with the chronic and cumulative neurotoxicity make the long-term therapy with thalidomide difficult3). Additionally, the hepatotoxicity in patients with a chronic hepatitis B virus (HBV) infection has not been clarified. We experienced mortality as the result of the acute exacerbation of chronic hepatitis B following the administration of thalidomide in a patient with a relapsed multiple myeloma.
CASE REPORT
This 50-year-old male patient was known to be HBsAg-positive since 1995. He was diagnosed with multiple myeloma at stage IIIA on November 1999. His liver function was normal, HBeAg/Anti-HBe were negative/positive and serum M protein was the monoclonal IgG lambda type. A bone marrow aspirate revealed 50% of the plasma cells and bone marrow cytogenetics of 43, XY, ŌłÆ8, ŌłÆ18, ŌłÆ20[1]/47, XY, +21[1]/46, XY[4], After three cycles of VAD chemotherapy (VAD: vincristine, adriamycin and dexamethasone), a partial response was achieved. He then underwent an autologous stem cell transplantation (SCT) on April 2000, which resulted in a complete remission. His disease recurred on March 2001, so he received the second autologous SCT two months later.
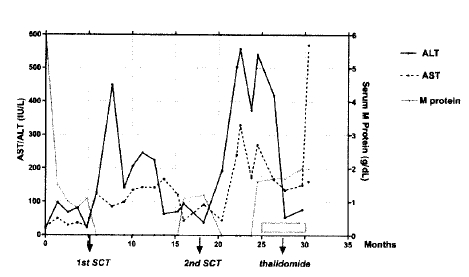
He suffered two episodes of transient transaminitis 3 and 4 months after the two successive autologous SCT (Figure 1). At those times, his HBeAg, HBV-DNA and PCR were all negative, with a fatty liver being the only abdominal image finding. He denied the consumption of neither herbal nor any traditional medications. Just before the second episode of transaminitis, he had consumed maximal five bottles of beer per day. After the several months of watchful follow-up, the transaminitis spontaneously resolved. He was also diagnosed with diabetes mellitus at the time of the first relapse and continued the administration of an oral hypoglycemic agent.
After a 3 months of disease-free interval from the second SCT, he showed the reappearance of serum M protein, but without infiltration of plasma cells in the bone marrow. Salvage treatment was started with thalidomide at a dose of 400 mg per day p.o in January 2002. Five months after initiation of the thalidomide therapy, he experienced sudden dizziness, general malaise and jaundice. There was a marked rise in his bilirubin and transaminases (Figure 1). His serum total/direct bilirubin, ALT/AST, alkaline phosphatase and ╬│-GT were 18.1/10.3 mg/mL, 161/569 U/L, 353 (70ŌĆō290) U/L and 100 (0ŌĆō75) U/L, respectively. The HBV DNA titer increased to 1,641 pg/mL, but the HBeAg was negative. Severe thrombocytopenia (29,000/╬╝L) and marked prolongation of PT/aPTT (INR 3.70/70 sec), with nasal bleeding, were immediately supported with transfusion. A bone marrow aspiration and biopsy revealed no plasma cells. His Sserum M component was stationary at 2 g/dL from 15 days previously. The thalidomide was stopped and lamivudine initiated, with supportive care. He deteriorated to coma on the third day of hospitalization, and died on the fourth hospital day. Klebsiella pneumoniae were cultured in his blood specimens. Serology for the Herpes Simplex and Epstein-Barr Viruses, and Cytomegalovirus were all-negative.
DISCUSSION
The prevalence of HBV in Korea was 5.7%4) in 1990s. After the initiation of routine screening and a vaccination program at birth in the 1980s, its prevalence markedly dropped. However, in middle or old age the prevalence is still high, and the concomitantly morbid cases with a cancer, such as multiple myeloma, are not rare in these aged groups.
Our case suffered two episodes of transient transaminitis during the disease-free period following two successive autologous stem cell transplantations. Multiple myeloma and HBV infection themselves can develop liver disease independent of the medication-related toxicity. However, the initiation of hepatic dysfunction during disease-free periods (Figure 1), and negative HBeAg, HBV DNA and PCR led us to believe that both episodes were due to toxic hepatitis of unknown etiology or alcohol-induced. Lau GK et al reported 15 episodes of transient hepatitis, which occurred at a median of 4 months after BMT in 24 patients with positive HBsAg. The etiologies of the above events were HBV reactivation (68%), relapse of underlying diseases (13%), sepsis (7%), and indeterminate (13%)5).
Biliary sepsis could not be excluded as the cause of the last hepatic dysfunction with severe jaundice, as the ductal dilatation was not evaluated due to familial refusal. However, the relatively milder increases in the serum alkaline phosphatase and ╬│-GT than in the transaminases, and the newly developed viremia helped us assume that the acute exacerbation of HBV had preceded the bacterial sepsis. The exact mechanisms of the exacerbation of the chronic hepatitis B in this patient were difficult to evaluate. The denial of any hepatotoxic agents, and the stationary disease status of the multiple myeloma, without involvement of the bone marrow, excluded toxic or tumor-mediated hepatic injuries. Thalidomide-induced T cell proliferation might evoke the destruction of HBV-infected hepatocytes. Thalidomide may have a dual immunomodulatory effect; down-regulation of TNF-╬▒6) and stimulation of IL-12-secreting T cells. Thalidomide has been proven to co-stimulate human T cells in vitro, preferentially the CD8+ T cell subset7). The later effect may have been prominent in this case.
Thalidomide-associated hepatitis has been reported in only one case with chronic hepatitis C8). A 58-year-old woman showed temporal deterioration in liver function 6 days after the administration of thalidomide for plasma cell leukemia, which improved within a week of thalidomide cessation. The elevation of the Hepatitis C Virus (HCV) RNA was suggestive of a causal relationship between the hepatitis and thalidomide. Severe thrombocytopenia was also combined as in our case, which normalized with the improvement of liver function. It is possible that thalidomide acts as a direct hepatotoxin, or as an immunomodulator, which alters the activity of chronic hepatitis C.
Conversely, Raufman et al. reported normalization of the serum ALT levels during 2 yearsŌĆÖ of thalidomide therapy in a patient with a relapsed myeloma and chronic hepatitis C9). In this case, the transient increase of ALT preceded the normalization. The mechanism of this phenomenon was thought to be the inhibition of TNF-╬▒. Another case the co-infection with disseminated Herpes Simplex and Varicella-Zoster Viruses during thalidomide treatment should be cautiously interpreted as the possible immunosuppressive effects of thalidomide10).
These conflicting data can cause clinicians to hesitate about the use of thalidomide in patients with chronic viral hepatitis. With the extension of the clinical indications of thalidomide, more careful monitoring of the viral burden and prophylactic prescription of antiviral agents during the thalidomide therapy should be recommended in patients with underlying viral hepatitis or liver injury.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





