INTRODUCTION
Adhesion molecules are related to cell-to-cell interaction and inflammatory interaction1). In addition, adhesive interactions between tumor cells and adjacent cells and/or extracellular matrix are known to be important in tumor growth2).
One of these adhesion molecules is the Intercellular Adhesion Molecule-1(ICAM-1), which is a 90kD type-1 trans-membrane glycoprotein, and is known to result in various interactions by binding with the ligands, β2 integrin LFA-1 (CD11a/CD18) and MAC-1(CD11b/CD18) expressed in lymphocytes3,4).
ICAM-1 is expressed on hematopoietic cells such as tissue macrophages, monocytes, activated T-cells, B-cells, tonsils, and lymph nodes, as well as on nonhematopoietic cell surfaces such as vascular endothelial and smooth muscle cells, thymus epithelial cells, and fibroblasts5,6).
Studies reported an increased expression of ICAM-1 in colon cancer7), bladder cancer8,9), lung cancer10,11), melanoma12), pancreatic cancer13,14), and hepatocellular carcinoma15). The adhesion molecules such as ICAM-1 and vascular cell adhesion molecule-1 (VCAM-1) are implicated in metastasis and poor prognosis16,17).
Thus, we examined the relationship between serum ICAM-1 and histologic classification, TNM stage, and survival time in the lung cancer patient.
MATERIALS AND METHODS
1. SUBJECTS
The study was done with data collected from 84 admitted patients who were diagnosed with lung cancer according to bronchoscopy, fine needle aspiration biopsy, and sputum cytology at Kangbuk Samsung hospital from January 1, 2000 to December 31, 2001. The subjects included 67 men and 20 women, whose average age was 60.5±10.8 years. According to histopathologic classification, there were 66 cases of non-small cell lung cancer (NSCLC) and 18 cases of small cell lung cancer (SCLC). NSCLC cases included 26 cases of squamous cell carcinoma, 34 cases of adenocarcinoma, and 6 cases of other cancers (2 cases of large cell carcinoma, 2 cases of carcinoid and 2 cases of bronchioloalveolar carcinoma). As for the pathologic stages of NSCLC, 1 case was in stage I, 1 was stage IIA, 3 were stage IIB, 8 were stage IIIA, 25 were stage IIIB, and 28 were stage IV. Thus, 11 cases were in a stage earlier than IIIA and their average age was 59.3±6.8 years. Fifty five cases were of stage greater than IIIB and their average age was 59.8±10.9 years. In SCLC cases, 4 patients with an average age of 60.8±13.0 years were in the limited stage, and 14 patients whose average age was 63.9±12.6 were in extended stage. Fifty nine patients were smokers with an average age of 61.0±10.3 years, and 25 patients were non-smokers with an average age of 59.2±12.1 years.
2. METHODS
Blood samples were collected from the 84 patients who were diagnosed with lung cancer using bronchoscopy, fine needle aspiration biopsy and sputum cytology. The samples were kept at room temperature for more than 30 minutes and each sample was centrifuged for 10 min at 1000×g and kept at −70°C until use. The concentration of serum ICAM-1 was measured using the ELISA kit from the R&D system. The reagent, standards, samples, and controls were prepared after the serum was diluted to 1:20. After placing 100 μL of diluted conjugate into each well, 100 μL of standard, control and sample was placed into the well. Incubation was done at room temperature for 1.5 h, aspiration and washing were repeated 6 times, and 100 μL of substrate was added. After incubation was done at room temperature for 30 min, 100 μL of stop solution was added and color development was observed within 30 minutes at 450 nm using a plate reader. Then, the degree of color development was compared with that of standard solution to calculate the concentration of serum ICAM-1 in the sample.
When the concentration of serum ICAM-1 was measured in blood samples collected from 131 normal individuals using the R&D system, the average concentration was 211 ng/mL, and average±2 SD was 115–306 ng/mL. Thus, we divided the concentration of serum ICAM-1 into the high concentration group (≥306 ng/mL) and low concentration group (<306 ng/mL) and compared the prognosis of each group.
3. STATISTICAL ANALYSIS
Data was analyzed using SPSS (version 11.0) statistical package. Each value was compared using the one-way ANOVA test. Bonferroni test was used as the post hoc test. The measured results were expressed in average (±SD). The rate of survival was determined using the Kaplan-Meier method. Statistical significance was determined at p<0.05.
RESULTS
1. Serum ICAM-1 concentrations according to the stage of cancer
Serum ICAM-1 concentrations according to the stage of cancer were determined by TNM staging in NSCLC patients (n=66). No significant difference among the different stages were present; the concentration was 323.3 ng/mL in stage I, 199.1 ng/mL in stage IIA, 363.6±85.4 ng/mL in stage IIB, 308.1±87.4 ng/mL in stage IIIA, 318.5±132.0 ng/mL in stage IIIB, and 327.7±144.4 ng/mL in stage IV (p value=0.91). The serum ICAM-1 concentration was 297.7±80.5 ng/mL in stages lower than IIIA and was 333.7±153.9 ng/mL in stages greater than IIIB, showing no significant difference (p value=0.45).
Likewise, no significant difference was present in SCLC patients (n=18), where the concentration was 385.0±141.0 ng/mL in the limited stage and 310.8±149.4ng/mL in the extended stage (p value=0.39) (Table 1).
2. Serum ICAM-1 concentrations according to histological type
The serum ICAM-1 concentration was 327.7±144.4 ng/mL in NSCLC (n=66) and was 327.3±146.9 ng/mL in SCLC (n=18), showing no significant difference between the two groups (p value=0.68).
In the case of NSCLC, this concentration was 356.9±130.9 ng/mL in adenocarcinoma (n=34), 321.4±144.5 ng/mL in squamous cell carcinoma (n=26), and 189.9±157.9 ng/mL in other cancers (n=6), showing no significant difference in serum ICAM-1 concentration between adenocarcinoma and squamous cell carcinoma (p value=0.32). This concentration in other cancers including large cell carcinoma (n=2), carcinoid (n=2), and bronchioloalveolar carcinoma (n=2) did not have statistical significance due to small sample size(Table 1).
3. Comparison of serum ICAM-1 concentration between smokers and nonsmokers
The concentration of serum ICAM-1 was 322.4±127.6 ng/mL in smokers (n=59) and 340.2±179.5 ng/mL in non-smokers (n=25), showing no significant difference between the two groups (p value=0.61) (Table 1).
4. Relationship between serum ICAM-1 concentration and prognosis
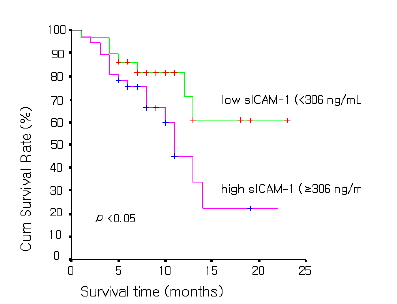
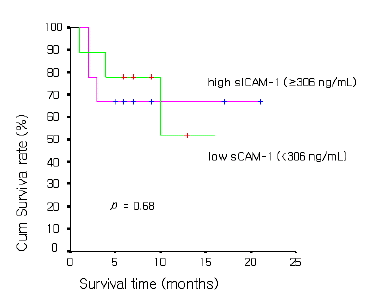
As for prognosis according to serum ICAM-1 concentration in NSCLC, the overall survival time was 11.9±1.6 months in 29 cases of the high serum ICAM-1 concentration group (≥306 ng/mL) and was 17.4±7.8 months in 46 cases of the low concentration group (<306 ng/mL), showing that the overall survival time was significantly longer in the low concentration group (p value<0.05) (Figure 1). In SCLC, the overall survival time was 14.8±2.9 months in 9 cases of the high sICAM-1 concentration group (≥306 ng/mL) and was 11.4±2.4 months in 9 cases of the low concentration group (<306 ng/mL), showing no significant difference between the two groups (p value=0.12) (Figure 2.)
DISCUSSION
Most cells in the body closely come in contact with other adjacent cells, basal membrane, or extracellular matrix. Adhesion molecules are known to mediate this contact between cell-to-cell or cell-to-extracellular matrix18). One of these adhesion molecules is ICAM-1, which is a 90kD type-1 trans-membrane glycoprotein. It is known that this molecule participates in inflammatory interaction and tumor progression by binding with the ligands, β2 integrin LFA-1 (CD11a/CD18) and MAC-1 (CD11b/CD18), expressed in lymphocytes3,4).
Tsujisaki et al reported that not only tumor cells, but also, fibroblasts surrounding the cancerous lesion would strongly express ICAM-1 when tumor cells themselves produce cytokines by activating T-cells and the level of sICAM-1 is increased at this time by being extracellularly released proportional to the level of surface ICAM-119). An increased expression of sICAM-1 was reported in colon cancer7), bladder cancer8,9), lung cancer10,11), melanoma12), pancreatic cancer13, 14), and hepatocellular carcinoma15). It is also known that increased expression of serum ICAM-1 is related with metastasis and poor prognosis16, 17). We examined the effectiveness of serum ICAM-1 as an indicator for disease progression and prognosis in patients with lung cancer.
Taguchi et al. reported that tumor cells and surrounding normal cells increase the expression and secretion of ICAM-1 by lung cancer cells, causing an increase in the secretion of various cytokines. Thus, the level of sICAM-1 would increase as the disease progresses. However, we could not find significant difference according to disease stages in NSCLC and SCLC20). In NSCLC, Schardt et al. reported that the concentration of sICAM-1 has shown greater increases in large cell carcinoma compared to other histological types21). On the other hand, Sprenger et al. reported that no relationship exists between histologic differences and serum ICAM-1 concentration in lung cancer22). We found no significant relationship between histological classification and serum ICAM-1 concentration in this study. The level of serum ICAM-1 could be increased in patients with not only lung cancer, but also, benign lung diseases such as tuberculosis, pneumonia, acute bronchitis, chronic asthma, and chronic obstructive lung disease22) and in the presence of risk factors of atherosclerosis such as diabetes, dyslipidemia, smoking and obesity. Thus, it would not be appropriate to determine prognostic factors of lung cancer according to serum ICAM-1 level23–25).
Various stimuli such as hypoxia or inflammatory cytokines would induce functional failure of endothelial cells and expression of adhesion molecules such as ICAM-1 and VCAM-1, which work on cells and endothelial cells26). Smoking, a well known risk factor of lung cancer, could increase the expression of ICAM-1 by inducing functional failure of endothelial cells, regardless of the progression of lung cancer. Thus, we believe that we could not find significant difference in serum ICAM levels between smokers and non-smokers27, 28).
Kageshita et al. reported that the serum ICAM-1 level could be used as a non-invasive marker of liver metastasis and that the level is related to disease progression in malignant melanoma17). Other authors reported that survival time decreased as serum ICAM-1 level increased in patients with malignant melanoma; thus, serum ICAM-1 levels has been proposed to be a marker influencing prognosis29). In NSCLC patients, we found that survival time was significantly higher in the low serum ICAM-1 group. No mechanism of prognosis according to serum ICAM-1 level has been elucidated. Because increased levels of serum ICAM-1 cause increased levels of cytokines, we have hypothesized that the increased levels of serum ICMA-1 cause tissue damage, promoting tumor migration and extravasation, and eventually inducing metastasis17, 30). In SCLC patients, no significant difference was seen between patients with low levels of serum ICAM-1 and those with high levels of serum ICAM-1. However, further studies are needed in the future because the number of SCLC patients was small.
In this study, we found that serum ICAM-1 level showed no relationship to lung cancer stage and histological type, and prognosis was significantly poorer in NSCLC patients with high serum ICAM-1 concentration. Hence, additional studies are needed with more patients to determine whether serum ICAM-1 level can be used as a marker for predicting the prognosis of NSCLC patients



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





