 |
 |
| Korean J Intern Med > Volume 19(1); 2004 > Article |
|
Abstract
Background :
Nonalcoholic fatty liver disease (NAFLD) has been more and more often regarded as a serious disorder, because nonalcoholic steatohepatitis (NASH), a part of NAFLD, may progress to the end stage of liver disease. Though an advanced age, obesity, diabetes mellitus (DM) etc. being not infrequent conditions in Korea, are known to exacerbate the severity of this disease, there are only a few Korean reports on this subject. The purpose of this study is to identify possible factors that might add up to the pathological severity of this disorder in Korean patients.
Methods :
Of 60 patients with steatosis found at liver biopsy, 43 NAFLD patients were reviewed retrospectively after exclusion of other liver diseases.
Results :
The cases of steatosis were mild, moderate, and severe in 9, 10, and 24 patients, respectively. The degree of necroinflammatory activity was mild, moderate, and severe in 33, 9, and 1 patients, respectively. There were no established factors directly related to these classes. As to fibrosis, the cases were classified as none, mild, moderate, severe, and cirrhotic in 9, 11, 16, 7, and 0 patients, respectively. The stage of fibrosis correlated with the age (p<0.001), BMI (body mass index) (p=0.032), and the platelet count (p=0.009), but the presence of NASH was associated only with BMI (p=0.002) and obesity (p=0.001).
Conclusion :
It seems that there are no factors that are directly related to the degree of steatosis or necroinflammatory activity. BMI seems to be a unique factor directly related to both the severity of fibrosis and the presence of NASH. The age and the platelet count are factors that are directly related to the degree of fibrosis but not to the presence of NASH.
Nonalcolic fatty liver disease (NAFLD) is a disorder of a wide spectrum of symptoms including those of simple steatosis without any inflammation or fibrosis, steatosis with nonspecific inflammation, and steatosis accompanied by inflammation and fibrosis, namely nonalcoholic steatohepatitis (NASH)1–3). NASH is a kind of hepatitis with pathologic findings similar to those of alcoholic hepatitis in nonalcoholics1, 2). Since Ludwig and colleagues for the first time described this disease in 19804), the seriousness of this disorder has been being increasingly recognized, because it may progress to cirrhosis, hepatic failure, and even hepatocellular carcinoma5–8). Several conditions such as an advanced age, obesity, DM or insulin resistance, hyperlipidemia, and female gender have been reported as predisposing factors in this disorder1,4,9–12). And in several articles3, 6, 13–15), a few conditions, such as an advanced age, type II DM, obesity, and increased AST/ALT ratio have been reported to be the conditions that can lead to the development of severe fibrosis. There have been only few Korean reports16, 17) on this subject, and the results are not fully consistent with those of other reports. The aim of this study was to identify possible factors that may influence the pathological severity, such as steatosis, inflammation, and fibrosis in Korean NAFLD patients.
Reports of 933 liver biopsies which were done between year 1998 and 2002 were analysed retrospectively. Different degrees of steatosis were found in 60 patients at microscopic examination, and, finally, 43 patients with NAFLD were reviewed after clinical exclusion of liver diseases, such as alcoholic liver disease, viral hepatitis, autoimmune hepatitis, drug-induced hepatitis, primary biliary cirrhosis, Wilson’s disease, hemochromatosis, biliary obstruction, secondary nonalcoholic steatohepatitis, etc. The alcohol intake below 40 g/week was applied as a criterion for exclusion of alcoholic liver disease. Patients who had experienced gastrointestinal surgery or taken drugs1, 3) which may induce hepatic steatosis, such as corticosteroids, estrogens, methotrexate, tetracycline, calcium channel blockers, or amiodarone were excluded.
Liver biopsy was done with employment of ultrasonography in all patients, stained with hematoxylin-eosin and Masson’s trichrome. All biopsy specimens were reviewed again by one pathologist. The grading and staging system proposed by Brunt and et al.18) was used to classify the severity of the disease; i.e., the degree of steatosis was graded as 1 (mild), 2 (moderate), and 3 (severe), based on the percent of hepatocytes in the given biopsy specimen. The necroinflammatory activity was also graded as 1 (mild), 2 (moderate), and 3 (severe). Because there was only one patient whose necroinflammatory activity was graded 3 in this study, the comparison was done between two groups, “grade 1” and “grade 2 or more,” for the purpose of the statistical processing. Fibrosis was classified into stages 0 (none), 1 (pericellular or perisinusoidal), 2 (periportal), 3 (bridging), and 4 (cirrhosis). The disease entity “NASH” was considered only when, at least, minimal fibrosis was present, and other cases were considered as “simple steatosis” regardless of the necroinflammatory activity. Presence of Mallory’s hyaline was reported in all specimen. Age, gender, body mass index (BMI), presence of DM or hypertension, serum or blood tests, such as the white blood cell count (WBC), hemoglobin (Hb), platelets, total protein, albumin, total and direct bilirubin, alkaline phosphatase (ALP), aspartate transaminase (AST), alanine transaminase (ALT), AST/ALT ratio, triglyceride (TG), cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL), prothrombin time (PT) prolongation, partial thromboplastin time (PTT), type IV collagen, and hyaluronic acid level were reviewed. The patient was considered obese when BMI exceeded 25 and DM case, if there was a documented administration of oral hypoglycemic agents or insulin, with the fasting glucose level exceeding 125 mg/dL on 2 occasions, or random glucose level exceeding 200 mg/dL. Hypertension was considered when the patient had been taking antihypertensive medicine or when diastolic blood pressure exceeding 90 mmHg or systolic blood pressure exceeding 140 mmHg was noted more than on 2 separate days.
The SPSS statistical package 11.0 (SPSS Inc., Chicago, IL., U.S.A.) was used for the statistical analysis. All the data were summarized by mean±SD (standard deviation) for continuous variables and by frequency or percentage for categorical variables. The values of the variables were compared among each grades of steatosis and the necroinflammatory activity. The comparison was also done in respect of each stage of fibrosis and between the groups of simple steatosis and NASH. The univariate analysis was performed using Mann-Whitney test for comparison of two groups and Kruscal-Wallis test for more than two groups. Chi-square test was used for comparison of the frequency data. Values of p less than 0.05 were considered as “significant” and values of p between 0.05 to 0.1 were considered as “tending to significant” statistically. The multiple regression analysis was used to identify the independent effects of significant variables on steatosis, the necroinflammatory activity, and fibrosis.
The main clinical and laboratory data of the patients of the study are summarized in Table 1. Thirty four (79.1%) patients were male. The mean age was 32.9 years. Mean BMI was 26.8, and the obese patients, whose BMI exceed 25.0, were thirty (69.8%). Three (7.0%) patients had DM and six (14.0%) patients had hypertension. Only three (7.0%) patients had AST/ALT ratio above 1.
The biopsy findings are summarized in Table 2. The grades of steatosis were 1 in 9 (20.9%), 2 in 10 (23.3%), and 3 in 24 (55.8%) patients, respectively. The grades of the necroinflammatory activity were 1, 2, and 3 in 33 (76.8%), 9 (20.9%), and 1 (2.3%) patients, respectively. There were 9 (20.9%) simple steatosis and 34 (79.1%) NASH patients. The ostages of fibrosis were 0 in 9 (20.9%), 1 in 11 (25.6%), 2 in 16 (37.2%), and 3 in 7 (16.3%) patients, respectively. There were no patients with fibrosis stage 4. Mallory bodies were found in 7 (16.3%) patients.
In the univariate analysis (Table 3), there was a significant difference in the direct bilirubin concentration among the 3 grades groups of steatosis (p=0.025), but the mean levels were within the normal range. The degree of steatosis by DM (p=0.076), total bilirubin concentration (p=0.055), AST (p=0.057), and serum cholesterol level (p=0.064). None of these variables were significant independently in the multivariate analysis.
Though the patients of the advanced age (p=0.082) and low hemoglobin concentration (p=0.056) showed a tendency to have a more severe activity, there were no independent factors which could be regarded as directly associated with the degree of the necroinflammatory activity (Table 4).
Age (p=0.034), BMI (p<0.001), obesity (p=0.011), and the platelet count (p=0.001) showed a significant correlation with the stage of fibrosis in the univariate analysis (Table 5). Of them, age (p=0.032), BMI (p<0.001), and the platelet count (p=0.009) also showed a significant correlation with the stage of fibrosis in the multivariate analysis (Table 6). The significant difference results from the different values of the stage 3 group by the different values of the stage 3 group (Figure 1).
The pathologic findings of NASH are similar to those in alcoholic hepatitis1), but there are some inconsistencies in the diagnosis of this disease due to different diagnostic criteria applied in the studies, on which the reports have been published. Although some studies used expanded criteria, which require only nonspecific inflammation combined with steatosis, to diagnose NASH3,5, 11,19,20), other studies used criteria which impose strictly specific conditions, such as hepatocyte ballooning degeneration, fibrosis, and neutrophilic infiltration with or without Mallory hyaline2, 4, 6, 9, 10). In previously published two Korean studies16,17), the expanded criteria were used to diagnose NASH. In this study, we used only strictly specific criteria to diagnose NASH, because recent studies report that the prognosis of NAFLD patients differs considerably in respect to the presence of fibrosis1–3). We considered three factors proposed by the Brunt et al.18) to describe the histology of NAFLD as variables representing the severity of this disease. They are the grades of steatosis, the grades of the necroinflammatory activity, and the stage of fibrosis. Then several clinical and laboratory factors which effect the degree of each variable were analyzed.
In general, several conditions, such as obesity, DM or insulin resistance, hyperlipidemia, hypertension, and other metabolic disorders have been known as causes of steatosis1, 4, 9–12, 22, 23). But in this retrospective study, the influence of the above factors on the development of steatosis couldn’t be analyzed, because there was no control group.
Although in many published articles23–25), the serum transaminase level, such as AST or ALT, has been reported to strongly correlate with the degree of the necroinflammatory activity in liver diseases, but report with different also exist26). In several reports, the correlation between the serum transaminase level and the necroinflammatory activity was poor or absent18, 27). Our study also shows no statistically significant factors, which effect the degree of the necroinflammatory activity.
Several conditions, such as obesity, type 2 DM or insulin resistance, an advanced age, and an increased AST/ALT ratio have been identified as risk factors of the development and progression of fibrosis in a number of previous reports3, 6, 13–15, 28, 29). In Korea, BMI was reported as a unique factor, which was associated with the development of fibrosis in both two previous reports,16, 17) and a low ALT level, besides BMI, was reported to add up to the severity of fibrosis in one report16). Our results are consistent with those of previous Korean reports in the stipulation that BMI is a unique factor, which influences the development of fibrosis. The cut-off BMI value of 26.0 was relevant for the diagnosis of NASH with the sensitivity and specificity of 76% and 89%, respectively. In terms of the severity of fibrosis, our results are not consistent with previous Korean reports in respect of age, platelet count, and ALT level. Though a low platelet count appeared to be related to the seventy of fibrosis, only one patient with severe fibrosis showed thrombocytopenia in our study. As the disease progresses from mild fibrosis to cirrhosis, the platelet count is known to decrease due to the disturbed thrombopoietin synthesis in viral hepatitis30). Further studies seems to be needed to assess the application of this phenomenon in NAFLD or NASH. The two laboratory variables, which are known to strongly correlate with the degree of fibrosis, type IV collagen and hyaluronic acid, were examined only in 18 patients. Being not statistically significant as they are, the values of these variables tend to be high in the fibrosis stage 3 group.
Figure 1.
The distribution of age (A), BMI (B), and platelet count (C) according to the stage of fibrosis. In all three above variables, the statistically significant differences were taken from radically different values of the stage 3 group.
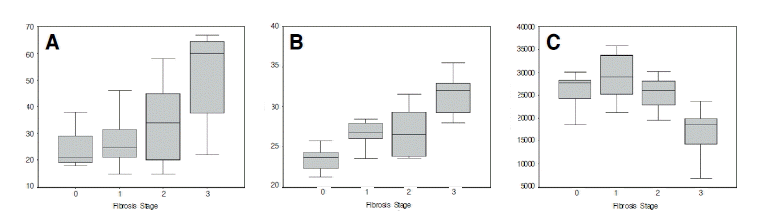
Figure 2.
ROC curve for diagnosis of NASH with BMI. Cut-off BMI value of 26.0 was relevant for theto diagnosis of NASH, with sensitivity and specificity of 76% and 89%, respectively (BMI : body mass index).
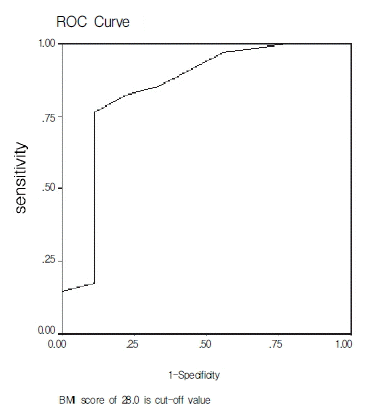
Table 1.
Patient demographics and basic laboratory data (n=43)
| Variables | Value/Number |
|---|---|
| Age (years) | 32.9 ±15.8 |
| Gender (Male) | 34 (79.1%) |
| BMI | 26.8±3.5 |
| Obesity (BMI>25) | 30 (69.8%) |
| Diabetes mellitus (+) | 3 (7.0%) |
| Hypertension (+) | 6 (14.0%) |
| WBC (/mm3) | 7206± 1437 |
| Hb (g/dL) | 14.7± 1.4 |
| Platelet (×1000/mm3) | 255± 63 |
| Protein (g/dL) | 7.5± 0.6 |
| Albumin (g/dL) | 4.7± 0.4 |
| T-Bilirubin (mg/dL) | 0.9± 0.4 |
| D-Bilirubin (mg/dL) | 0.3± 0.2 |
| ALP (IU/L) | 93.8± 51.8 |
| AST (IU/L) | 72.6± 39.4 |
| ALT (IU/L) | 133.8± 71.4 |
| AST/ALT ratio (>1) | 3 (7.0%) |
| TG (mg/dL) | 190.8± 93.7 |
| Chol (mg/dL) | 189.3± 31.6 |
| HDL (mg/dL) | 41.4± 8.1 |
| LDL (mg/dL) | 134.6± 34.7 |
| Type IV collagen (ng/mL)* | 4.3± 1.6 |
| Hyaluronic acid (ng/mL)* | 25.6± 5.2 |
Table 2.
Histologic data of the study group (n=43)
Table 3.
Univariate comparison among groups according to the degree of steatosis
|
Grade of steatosis
|
||||
|---|---|---|---|---|
| 1 | 2 | 3 | p value | |
| N | 9 | 10 | 24 | |
| Age (years) | 42.7 ±18.7 | 31.7±16.8 | 29.7 ±13.2 | 0.176 |
| Gender (Male) | 7 (77.8%) | 8 (80.0%) | 19 (79.2%) | 0.993 |
| BMI | 25.7 ±2.7 | 27.1 ±5.2 | 27.1 ±2.9 | 0.668 |
| Obesity (BMI>25) | 5 (55.6%) | 6 (60.0%) | 19 (79.2%) | 0.314 |
| Diabetes mellitus (+) | 2 (22.2%) | 1 (10.0%) | 0 (14.3%) | 0.076 |
| Hypertension (+) | 1 (11.1%) | 0 (0%) | 5 (20.8%) | 0.269 |
| WBC (/mm3) | 7054± 1799 | 7397± 1345 | 7184± 1382 | 0.838 |
| Hb (g/dL) | 14.1 ±1.2 | 14.8±1.5 | 14.9±1.4 | 0.317 |
| Platelet (×1000/mm3) | 243 ±77.4 | 244 ±68.1 | 264 ±55.3 | 0.822 |
| Protein (g/dL) | 7.8 ±0.8 | 7.5±0.7 | 7.5 ±0.5 | 0.555 |
| Albumin (g/dL) | 4.5 ±0.4 | 4.6±0.4 | 4.7 ±0.4 | 0.145 |
| T-Bilirubin (mg/dL) | 0.9 ±0.4 | 1.1 ±0.5 | 0.8 ±0.2 | 0.056 |
| D-Bilirubin (mg/dL) | 0.3 ±0.2 | 0.4 ±0.2 | 0.3 ±0.1 | 0.025 |
| ALP (IU/L) | 86.8 ±30.6 | 107.6±90.9 | 90.6 ±35.0 | 0.555 |
| AST (IU/L) | 72.6 ±49.0 | 57.0±37.7 | 92.1 ±51.3 | 0.057 |
| ALT (IU/L) | 126.2±71.1 | 125.1 ±85.3 | 140.3 ±67.8 | 0.760 |
| AST/ALT ratio (>1) | 0 (0%) | 0 (0%) | 3 (12.5%) | 0.279 |
| TG (mg/dL) | 169.2 ±73.8 | 168.2 ±100.9 | 205.6 ±97.0 | 0.460 |
| Chol (mg/dL) | 192.2 ±38.5 | 169.6±28.5 | 196.5 ±27.6 | 0.064 |
| HDL (mg/dL) | 42.2 ±2.9 | 40.1 ±7.4 | 41.8 ±9.5 | 0.901 |
| LDL (mg/dL) | 135.9 ±44.3 | 117.2±34.2 | 141.2 ±31.2 | 0.361 |
| Type IV collagen (ng/mL)* | 3.4 ±0.8 | 4.4±1.1 | 4.6 ±2.1 | 0.262 |
| Hyaluronic acid (ng/mL)* | 28.3 ±5.7 | 26.8 ±1.8 | 24.0 ±6.4 | 0.588 |
| Necroinflammatory grade | 1.33 ±0.71 | 1.20 ±0.42 | 1.25 ±0.44 | 0.952 |
| Fibrosis stage | 1.44 ± 1.13 | 1.40 ±1.08 | 1.33 ±0.96 | 0.972 |
| Mallory body (+) | 4 (25%) | 1 (9.1%) | 2 (28.6%) | 0.769 |
Table 4.
Univariate comparison among groups according to the degree of necroinflammatory activity
|
Grade of necroinflammatory activity
|
|||
|---|---|---|---|
| 1 | Above 2 | p value | |
| N | 33 | 10 | |
| Age (years) | 30.1 ±13.8 | 42.0 ±19.0 | 0.082 |
| Gender (Male) | 28 (84.8%) | 5 (15.2%) | 0.177 |
| BMI | 26.6 ±3.6 | 27.7 ±3.0 | 0.249 |
| Obesity (BMI>25) | 22 (66.7%) | 8 (80.0%) | 0.696 |
| Diabetes mellitus (+) | 1 (3.0%) | 2 (20.0%) | 0.130 |
| Hypertension (+) | 4 (12.1%) | 2 (20.0%) | 0.611 |
| WBC (/mm3) | 7110± 1309 | 7523± 1842 | 0.561 |
| Hb (g/dL) | 14.9±1.4 | 14.0±1.4 | 0.056 |
| Platelet (X1000/mm3) | 257 ±57.6 | 247 ±80.0 | 0.561 |
| Protein (g/dL) | 7.6 ±0.6 | 7.3 ±0.5 | 0.286 |
| Albumin (g/dL) | 4.7 ±0.4 | 4.6 ±0.3 | 0.944 |
| T-Bilirubin (mg/dL) | 0.9 ±0.4 | 0.8 ±0.2 | 0.452 |
| ALP (IU/L) | 87.6 ±37.6 | 114.2 ±82.9 | 0.341 |
| AST (IU/L) | 69.3 ±38.2 | 83.3 ±43.5 | 0.273 |
| ALT (IU/L) | 137.5 ±75.6 | 121.6 ±56.9 | 0.640 |
| AST/ALT ratio (>1) | 2 (6.1%) | 1 (10.0%) | 0.558 |
| TG (mg/dL) | 194.8 ±98.1 | 177.3 ±81.0 | 0.862 |
| Chol (mg/dL) | 189.5 ±30.4 | 188.6 ±36.9 | 0.854 |
| HDL (mg/dL) | 40.1 ±7.2 | 45.9 ±9.5 | 0.120 |
| LDL (mg/dL) | 137.0 ±32.7 | 126.8 ±42.2 | 0.307 |
| Type IV collagen (ng/mL)* | 4.6 ±1.4 | 3.4 ±2.4 | 0.382 |
| Hyaluronic acid (ng/mL)* | 26.5 ±2.6 | 22.6 ±10.4 | 0.645 |
| Steatosis grade | 2.33 ±0.82 | 2.40 ±0.84 | 0.832 |
| Fibrosis stage | 1.21 ±0.99 | 1.90 ±0.88 | 0.072 |
| Mallory body (+) | 4 (12.1%) | 3 (30.0%) | 0.325 |
Table 5.
Univariate comparison among groups according to the degree of fibrosis
|
Stage of Fibrosis
|
|||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | p value | |
| N | 9 | 16 | 11 | 7 | |
| Age (years) | 27.1 ±12.6 | 27.5 ±9.9 | 34.2 ±16.0 | 50.4 ±19.2 | 0.034 |
| Gender (Male) | 8 (88.9%) | 14 (87.5%) | 8 (72.7%) | 4 (57.1%) | 0.087 |
| BMI | 23.5±3.3 | 26.6 ±1.6 | 26.9 ±3.0 | 31.4±2.7 | 0.000 |
| Obesity (BMI>25) | 2 (22.2%) | 14 (87.5%) | 7 (63.6%) | 7 (100%) | 0.011 |
| DM (+) | 0 (0%) | 1 (6.3%) | 1 (9.1%) | 1 (14.3%) | 0.263 |
| Hypertension (+) | 1 (11.1%) | 2 (12.5%) | 0 (0%) | 3 (42.9%) | 0.306 |
| WBC (/mm3) | 7775± 876 | 6966± 1164 | 7719± 1586 | 6219± 1892 | 0.102 |
| Hb (g/dL) | 15.3±1.0 | 14.9±1.5 | 14.3±1.4 | 14.2±1.7 | 0.170 |
| Platelet (×1000/mm3) | 263± 55.7 | 290± 46.5 | 252± 37.5 | 168± 57.3 | 0.001 |
| Protein (g/dL) | 7.6±0.6 | 7.5 ±0.7 | 7.4±0.7 | 7.5±0.6 | 0.901 |
| Albumin (g/dL) | 4.7±0.3 | 4.6 ±0.4 | 4.7±0.4 | 4.6±0.4 | 0.603 |
| T-Bilirubin (mg/dL) | 0.9±0.5 | 1.0 ±0.4 | 0.8±0.1 | 0.9±0.3 | 0.787 |
| ALP (IU/L) | 99.0±41.1 | 91.2 ±67.1 | 95.5 ±51.1 | 90.3±28.3 | 0.526 |
| AST (IU/L) | 54.8 ±14.4 | 72.5 ±44.0 | 74.8±36.3 | 92.1 ±51.3 | 0.559 |
| ALT (IU/L) | 136± 73 | 125± 67 | 142± 65 | 137± 101 | 0.867 |
| AST/ALT ratio (>1) | 1 (11.1%) | 2 (12.5%) | 0 (0%) | 0 (0%) | 0.196 |
| TG (mg/dL) | 218± 116 | 158± 80 | 210± 96 | 192± 93 | 0.485 |
| Chol (mg/dL) | 193± 36 | 180± 30 | 207± 28 | 179± 29 | 0.122 |
| HDL (mg/dL) | 41.0±6.4 | 39.1 ±7.7 | 45.6±9.8 | 40.4±7.1 | 0.385 |
| LDL (mg/dL) | 142± 42 | 132± 36 | 146± 27 | 113 ±30 | 0.178 |
| Type IV collagen (ng/mL)* | 3.9±1.8 | 3.9 ±2.0 | 5.0±0.5 | 5.2 | 0.566 |
| Hyaluronic acid (ng/mL)* | 26.6±2.1 | 25.0 ±6.9 | 25.6±1.1 | 30.1 | 0.420 |
| Steatosis grade | 2.33± 0.87 | 2.37 ±0.81 | 2.45±0.82 | 2.14± 0.90 | 0.872 |
| Necroinflammatory grade | 1.00± 0.00 | 1.25± 0.45 | 1.27± 0.47 | 1.57± 0.79 | 0.207 |
| Mallory body (+) | 0 (0%) | 4 (25%) | 1 (9.1%) | 2 (28.6%) | 0.354 |
Table 6.
Multiple regression analysis about the association between the degree of fibrosis and related variables
| value | Regression Coefficient | SE of Regression Coefficient | F value | Significance |
|---|---|---|---|---|
| BMI | 0.536 | 0.030 | 26.978 | 0.000 |
| Age | 0.241 | 0.007 | 4.920 | 0.032 |
| Platlet | −0.300 | 0.000 | 7.640 | 0.009 |
| R2 | 0.583 |
Table 7.
Univariate comparison between groups according to the presence of fibrosis
|
Presence of fibrosis
|
|||
|---|---|---|---|
| − | + | p value | |
| N | 9 | 34 | |
| Age (years) | 27.1 ±12.6 | 34.4 ±16.3 | 0.164 |
| Gender (Male) | 8 (88.9%) | 26 (76.5%) | 0.657 |
| BMI | 23.5 ±3.3 | 27.7±3.0 | 0.001 |
| Obesity (BMI>25) | 2 (22.2%) | 28 (82.4%) | 0.001 |
| Diabetes mellitus (+) | 0 (0.0%) | 3 (8.8%) | 1.000 |
| Hypertension (+) | 1 (11.1%) | 5 (14.7%) | 1.000 |
| WBC (/mm3) | 7775± 876 | 7056± 1526 | 0.114 |
| Hb (g/dL) | 15.3±1.0 | 14.6±1.5 | 0.066 |
| Platelet (×1000/mm3) | 263 ±55.7 | 253 ±64.9 | 0.687 |
| Protein (g/dL) | 7.6 ±0.6 | 7.5 ±0.6 | 0.491 |
| Albumin (g/dL) | 4.7 ±0.3 | 4.6 ±0.4 | 0.641 |
| T-Bilirubin (mg/dL) | 0.9 ±0.5 | 0.9 ±0.3 | 0.751 |
| ALP (IU/L) | 99.0 ±41.1 | 92.4 ±54.7 | 0.269 |
| AST (IU/L) | 54.8 ±14.4 | 77.3 ±42.6 | 0.256 |
| ALT (IU/L) | 135.8 ±72.8 | 133.3 ±72.1 | 0.731 |
| AST/ALT ratio (>1) | 1 (11.1%) | 2 (5.9%) | 0.515 |
| TG (mg/dL) | 217.7±116.5 | 184.1 ±88.3 | 0.509 |
| Chol (mg/dL) | 192.9 ±35.6 | 188.4 ±31.0 | 0.698 |
| HDL (mg/dL) | 41.0 ±6.4 | 41.5 ±8.6 | 0.983 |
| LDL (mg/dL) | 142.7 ±42.2 | 132.5 ±33.2 | 0.537 |
| Type IV collagen (ng/mL)* | 3.9 ±1.8 | 4.4 ±1.7 | 0.778 |
| Hyaluronic acid (ng/mL)* | 26.6 ±2.1 | 25.5 ±5.5 | 0.779 |
| Steatosis grade | 2.33 ±0.87 | 2.35 ±0.81 | 0.960 |
| Necroinflammatory activity | 1.00 ±0.00 | 1.32 ±0.54 | 0.067 |
| Mallory body (+) | 0 (0.0%) | 7 (16.3%) | 0.314 |
REFERENCES
1. Feldman M, Friedman LS, Sleisenger MH. Sleisenger & Fordtran’s gastrointestinal and liver disease. 7th ed. 1393. Philadelphia: Saunders, 2002.
2. Schiff ER, Orrell MF, Maddrey WC. Schiff's diseases of the liver. 9th ed. 1261. Philadelphia: Lippincott Williams & Wilkins, 2003.
3. Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 30:1356–13621999.


4. Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 55:434–4381980.


5. Bacon BR, Farahvash MJ, Janny CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastoenterology 107:1103–11091994.

6. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 116:1413–14191999.


7. Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology 36:1349–13542002.


8. Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, de Paolis P, Capussotti L, Salizzoni M, Rizzetto M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 123:134–1402002.


9. Diehl AM, Goodman Z, Ishak KG. Alcohol-like licer disease in nonalcoholics: a clinical and histologic comparison with alcohol-induced liver injury. Gastroenterology 95:1056–10621988.


11. Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology 11:74–801990.


12. Itoh S, Yougel T, Kawagoe K. Comparison between nonalcoholic steatohepatitis and alcoholic hepatitis. Am J Gastroenterol 82:650–6541987.

13. Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, Khalil L, Turpin G, Opolon P, Poynard T. Liver fibrosis in overweight patients. Gastroenterology 118:1117–11232000.


15. Sorbi D, Boynton J, Lindor KD. The ratio of aspartate aminotransferase to alanine aminotransferase: potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am J Gastroenterol 94:1018–10221999.


16. Kim SJ, Park JW, Kim MG, Kim HJ, Hong YH, Han SH, Kim JG, Yoo BC, Park SM. Clincial predictors reflecting the pathologic severity of nonalcoholic steaohepatitis in patients with nonalcoholic fatty liver. Korean J Gastroenterol 36:782–7922000.
17. Lee DS, Kweon KT, Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH, Bae HI. Predictive factors for the diagnosis of nonalcoholic steatohepatitis (NASH) and the degree of liver fibrosis in nonalcoholic fatty liver disease (NAFLD). Korean J Hepatol 9(Suppl 3):S14. 2003.
18. Brunt EM, Janney CG, di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steaohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94:2467–24741999.


19. Bonkovsky HL, Jawaid Q, Tortorelli K, le Clair P, Cobb J, Lambrecht RW, Banner BF. Non-alcoholic steatohepatitis and iron: increased prevalence of mutations of the HFE gene in non-alcoholic steatohepatis. J Hepatol 31:421–4291999.


20. George DK, Goldwum S, MacDonald GA, Cowley LL, Waker NI, Ward PJ, Jazwinska EC, Powell LW. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterlogy 114:311–3181998.

21. Adler M, Schaffner F. Fatty liver hepatitis and cirrhosis in obese patients. Am J Med 67:811–8161979.


22. Batman PA, Scheuer PJ. Diabetic hepatitis preceding the onset of glucose intolerance. Histopathology 9:237–2431985.


23. Cahen DL, van Leeuwen DJ, ten Kate FJ, Blok AP, Oosting J, Chamuleau RA. Do serum ALAT values reflect the inflammatory activity in the liver of patients with chronic viral hepatitis? Liver 16:105–1091996.


24. Paz MO, Brenes F, Karayiannis P, Jowett TP, Scheuer PJ, Thomas HC. Chronic hepatitis B virus infection: viral replication and patterns of inflammatory activity: serological, clinical and histological correlations. J Hepatol 3:371–3771986.


25. Luo JC, Hwang SJ, Lai CR, Lu CL, Li CP, Tsay SH, Wu JC, Chang FY, Lee SD. Relationship between serum aminotransferase levels, liver histologies and virological status in patients with chronic hepatitis C in Taiwan. J Gastoenterol Hepatol 13:685–6901998.

26. Haber MM, West AB, Haber AD, Reuben A. Relationship of aminotransferases to liver histological status in chronic hepatitis C. Am J Gastroenterol 90:1250–12571995.

27. Sonsuz A, Basaranoglu M, Ozbay G. Relationship between aminotransferase levels and histopathological findings in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 95:1370–13712000.


28. Pagano G, Pacini G, Musso G, Gambino R, Mecca F, Depetris N, Cassader M, David E, Cavallo-Perin P, Rizzetto M. Nonalcoholic steaohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology 35:367–3722002.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



