 |
 |
| Korean J Intern Med > Volume 39(5); 2024 > Article |
|
Abstract
Background/Aims
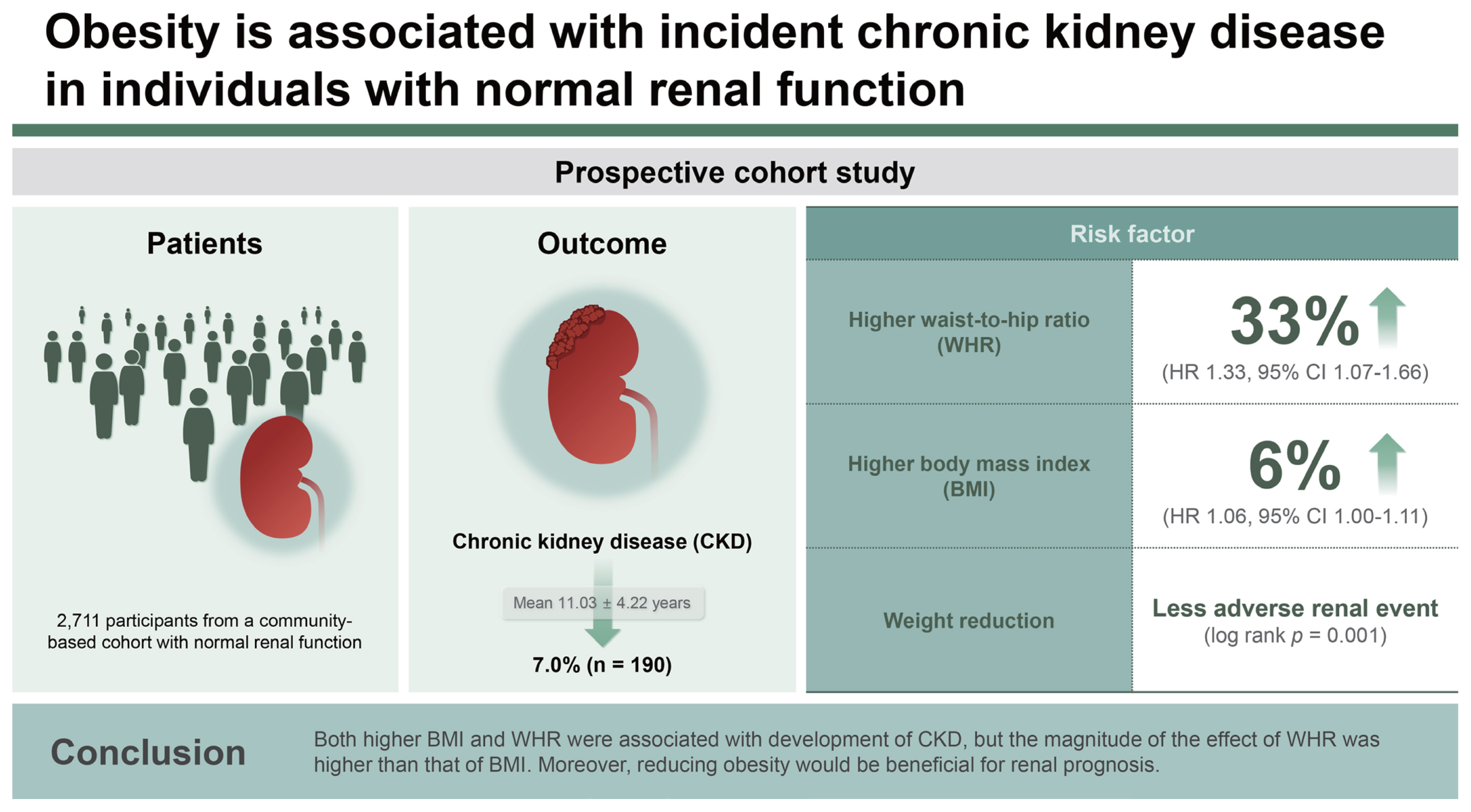
Obesity has known to be a modifiable risk factor associated with worse outcomes in chronic kidney disease (CKD), but few studies have examined the impact of obesity on CKD incidence in the general population. The purpose of this study was to investigate the role of body mass index (BMI) and waist-to-hip ratio (WHR) as predictors of incident CKD and to evaluate the impact of weight reduction on CKD prevention.
Methods
A total of 2,711 participants from a community-based cohort with normal renal function were prospectively analyzed. Among participants with obesity, we analyzed the change in WHR to evaluate the association of obesity reduction with CKD development.
Results
During a mean follow-up of 11.03 ┬▒ 4.22 years, incident CKD occurred in 190 (7.0%) participants. In the fully adjusted multivariable Cox proportional hazard models, the risk of incident CKD increased with higher BMI (hazard ratio, 1.06; 95% confidence interval, 1.00ŌĆō1.11; p = 0.033) and higher WHR (hazard ratio, 1.33; 95% confidence interval, 1.07ŌĆō1.66; p = 0.009). In the KaplanŌĆōMeier analysis, cumulative adverse renal events were significantly more common in the maintained obesity group than in the reduced obesity group (p = 0.001).
Obesity is a serious health problem worldwide with increasing prevalence, which has reached 40% in the United States and has been consistently rising in Asia [1]. Obesity is strongly associated with metabolic abnormalities, such as high blood pressure, dyslipidemia, high blood sugar levels, insulin resistance, and increased inflammation [2ŌĆō4], as well as with increased risk for cardiovascular and all-cause mortality [5,6]. In addition, obesity is associated with development of chronic kidney disease (CKD). A high body mass index (BMI) is strongly related to decrease in estimated glomerular filtration rate (eGFR) and progression to end-stage CKD [7,8]. Several mechanisms have been proposed to promote renal dysfunction in people with obesity, such as glomerular hyperfiltration, inflammation, and endothelial dysfunction [9,10]. CKD is a major public burden with rising global prevalence, imposing the need to identify and mitigate its associated risk factors.
The World Health Organization defines obesity as ŌĆ£an excessive fat accumulation [11].ŌĆØ Although BMI is a simple and useful tool, it tends to be affected by muscle mass. In addition, the BMI does not address regional adiposity. Thus, the waist-to-hip ratio (WHR), a measure of central obesity, may be a better tool for assessing obesity.
Obesity might be a representative modifiable risk factor for incident CKD. However, many studies have looked at obesityŌĆÖs impact on outcomes in CKD patients, thereŌĆÖs little research on how obesity in the general population can lead to CKD. In this study, we evaluated both BMI and WHR as risk factors for the development of incident CKD in the general population from the Korean Genome and Epidemiology Study (KoGES), which is a community-based prospective cohort study [12]. Moreover, to determine whether reducing obesity is beneficial for preventing renal dysfunction, we also compared renal function between individuals with maintained obesity and those with reduced obesity.
The KoGES is a large, prospective, community-based cohort study funded by the government. The detailed profile and methods concerning the development of KoGES have been previously described [12]. The present study included KoGES participants who were residents of Ansan, Korea. We excluded participants with an eGFR < 60 mL/min/1.73 m2 and those with missing follow-up data on serum creatinine levels and body weight.
All study participants voluntarily enrolled the KoGES after providing informed consent. This study was performed in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Chonnam National University Hospital (CNUH-EXP-2022-094).
All participants underwent comprehensive health examinations and filled out questionnaires on health and lifestyle at the time of enrollment. Serial health examinations and surveys were performed biennially from 2001 to 2018.
The following demographic and socioeconomic data were collected from the KoGES database: age, sex, alcohol intake, smoking status, and medical history. Diabetes mellitus (DM) and hypertension (HTN) were diagnosed based on the responses to the past medical history in the administered questionnaire. Information on alcohol intake and smoking status were obtained through questionnaires. Anthropometric parameters, including height, weight, waist circumference, and hip circumference, were measured by skilled study workers following standard methods. Blood pressure was measured after resting for more than 5 minutes in a sitting position.
Blood and urine samples were obtained after an 8-hour fasting and transported to a central laboratory (Seoul Clinical Laboratories, Seoul, Korea). The following biochemical data were determined: concentrations of blood urea nitrogen, creatinine, albumin, glucose, total cholesterol, triglyceride, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, glycated hemoglobin (HbA1c), hemoglobin, and C-reactive protein (CRP). Serum creatinine levels were measured using an isotope dilution mass spectrometry-traceable method. The eGFR was calculated using the CKD-EPI equation [13]. Proteinuria was measured categorically, and we defined proteinuria by classifying the ŌĆśnegativeŌĆÖ and ŌĆśtraceŌĆÖ group as group without proteinuria, and ŌĆś1 positiveŌĆÖ to ŌĆś4 positiveŌĆÖ as group with proteinuria. Insulin resistance was determined by the homeostasis model assessment of insulin resistance (HOMA-IR): Fasting glucose (mg/dL) ├Ś fasting insulin (╬╝IU/mL) / 405.
Obesity was defined both by the BMI and WHR. BMI was calculated by dividing the weight (kg) by the squared height (m2) and categorized as normal weight (BMI 18.5ŌĆō22.9 kg/m2), overweight (BMI 23ŌĆō24.9 kg/m2), or obesity (BMI Ōēź 25 kg/m2) according to the International Association for the Study of Obesity, International Obesity Task Force (2000), and Committee of Clinical Practice Guidelines and Korean Society for the Study of Obesity Guidelines [14]. WHR was calculated by dividing waist circumference by hip circumference. Obesity was defined as an WHR Ōēź 0.9 for men and Ōēź 0.85 for women [15].
The study outcome was incident CKD rate during the follow-up period, which was defined as a composite eGFR of < 60 mL/min/1.73 m2 for at least two consecutive measurements.
To evaluate the effect of obesity reduction on CKD development, we analyzed the changes in WHR from baseline to year 4. For this purpose, participants with an WHR Ōēź 0.9 for men and Ōēź 0.85 for women at both baseline and year 4 were defined as the maintained obesity group, while those with an WHR Ōēź 0.9 for men and Ōēź 0.85 for women at baseline and WHR < 0.9 for men and < 0.85 for women at year 4 were defined as the reduced obesity group. To determine the relationship between early WHR changes and subsequent CKD development, we only analyzed adverse renal events that occurred 4 years after the WHR assessment period.
Statistical analyses were performed using R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were summarized as mean ┬▒ standard deviation, and categorical variables as number (percentage). All data were tested for normality before analysis. A t-test or MannŌĆōWhitneyŌĆÖs U test was conducted to compare continuous variables, and the chi-square test was used to compare categorical variables. To analyze the changes in participantsŌĆÖ metabolic profile parameters during the follow-up period, we compared time-averaged values of fasting glucose, HbA1c, the HOMA-IR, CRP, and lipid profiles, which were analyzed as an average of the parameters examined at every follow-up visit.
Multivariable Cox regression models using cubic spline curves were used to determine the nonlinear association between the BMI or WHR and the risk of incident CKD. KaplanŌĆōMeier survival curves with log-rank tests and univariable Cox proportional hazards models were used to examine the effect of BMI or WHR on incident CKD. Multivariable Cox proportional hazards regression analysis was employed to identify independent risk factors for incident CKD. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated to estimate the risk of incident CKD. We examined the assumption of the proportional hazard in the Cox model using cox.zph() in R. Age- and sex-adjusted HRs were first calculated (model 1), and the results were further adjusted for HTN, DM, alcohol consumption, and smoking (model 2), and further for LDL cholesterol, hemoglobin, CRP, and baseline eGFR (model 3). For all analyses, a p value < 0.05 was considered to indicate statistical significance.
Of the 5,012 participants screened, 2,711 (age range: 40ŌĆō69 yr) were included in the final analysis (Fig. 1). The baseline characteristics of the total study population according to their BMI are presented in Table 1. Participants in the obesity group were predominantly older, had lower income, higher incidence of DM and HTN, higher HbA1c and triglyceride levels, and lower HDL cholesterol levels than those in the normal weight group.
During the follow-up period, the obesity group had persistently lower levels of HDL-cholesterol, and higher levels of fasting glucose, HbA1c, HOMA-IR, CRP, and triglycerides (Fig. 2).
During a mean follow-up of 11.03 ┬▒ 4.22 years, incident CKD occurred in 190 (7.0%) participants. In the fully adjusted Cox proportional hazards model (Table 2), BMI and WHR exhibited a positive correlation with the risk for incident CKD (Fig. 3). The risk for incident CKD increased with higher BMI (HR, 1.06; 95% CI, 1.00ŌĆō1.11; p = 0.033) and higher WHR (HR, 1.33; 95% CI, 1.07ŌĆō1.66; p = 0.009).
In the KaplanŌĆōMeier analysis for free-of-CKD probability (Fig. 4), the cumulative incidence of CKD development was significantly high (p < 0.001) in both obesity groups classified by BMI and WHR.
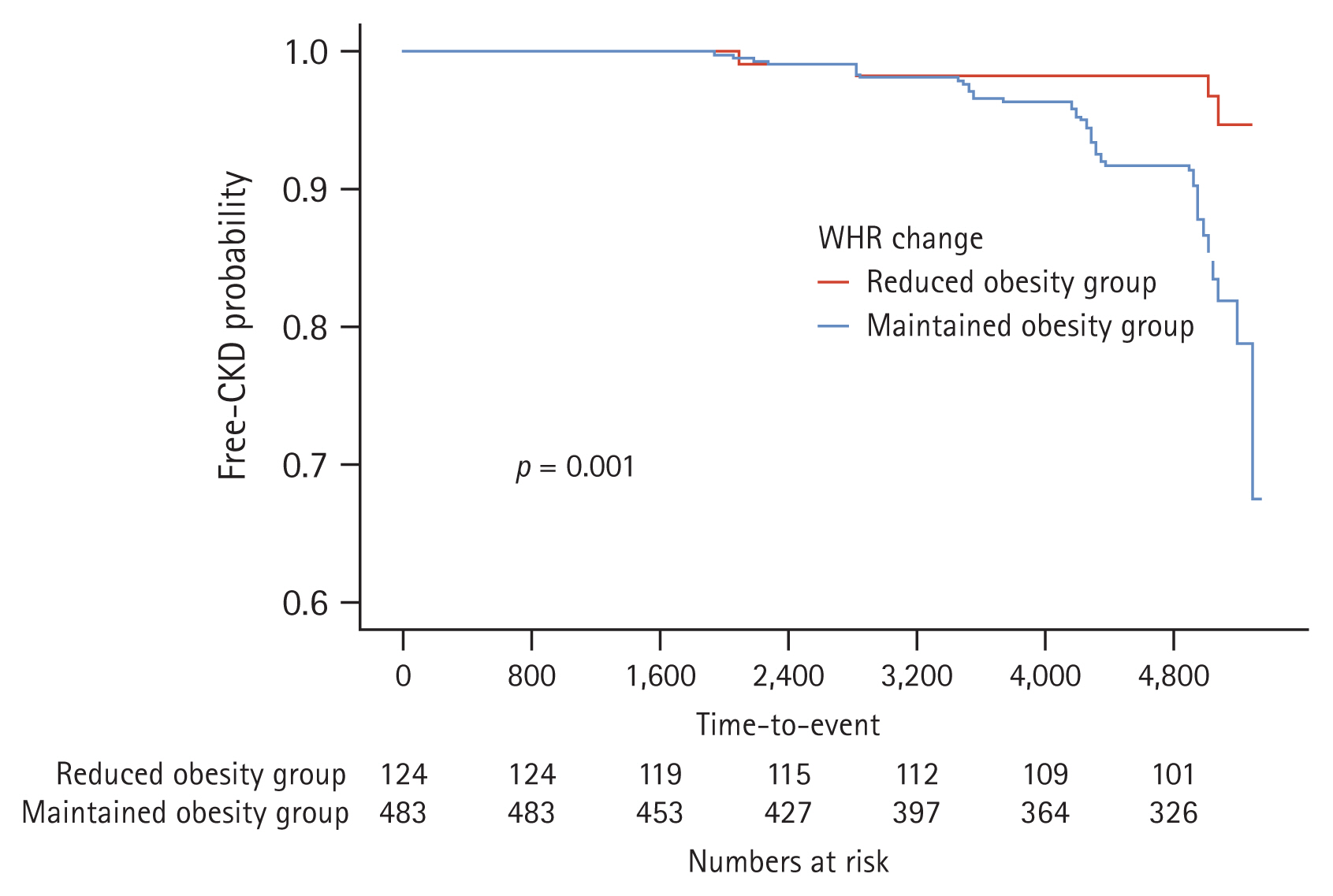
In the analysis of the effects of obesity reduction on incident CKD (Fig. 5), the KaplanŌĆōMeier curves showed that the rate of cumulative adverse renal events was significantly in the maintained obesity group than in the reduced obesity group (p = 0.001).
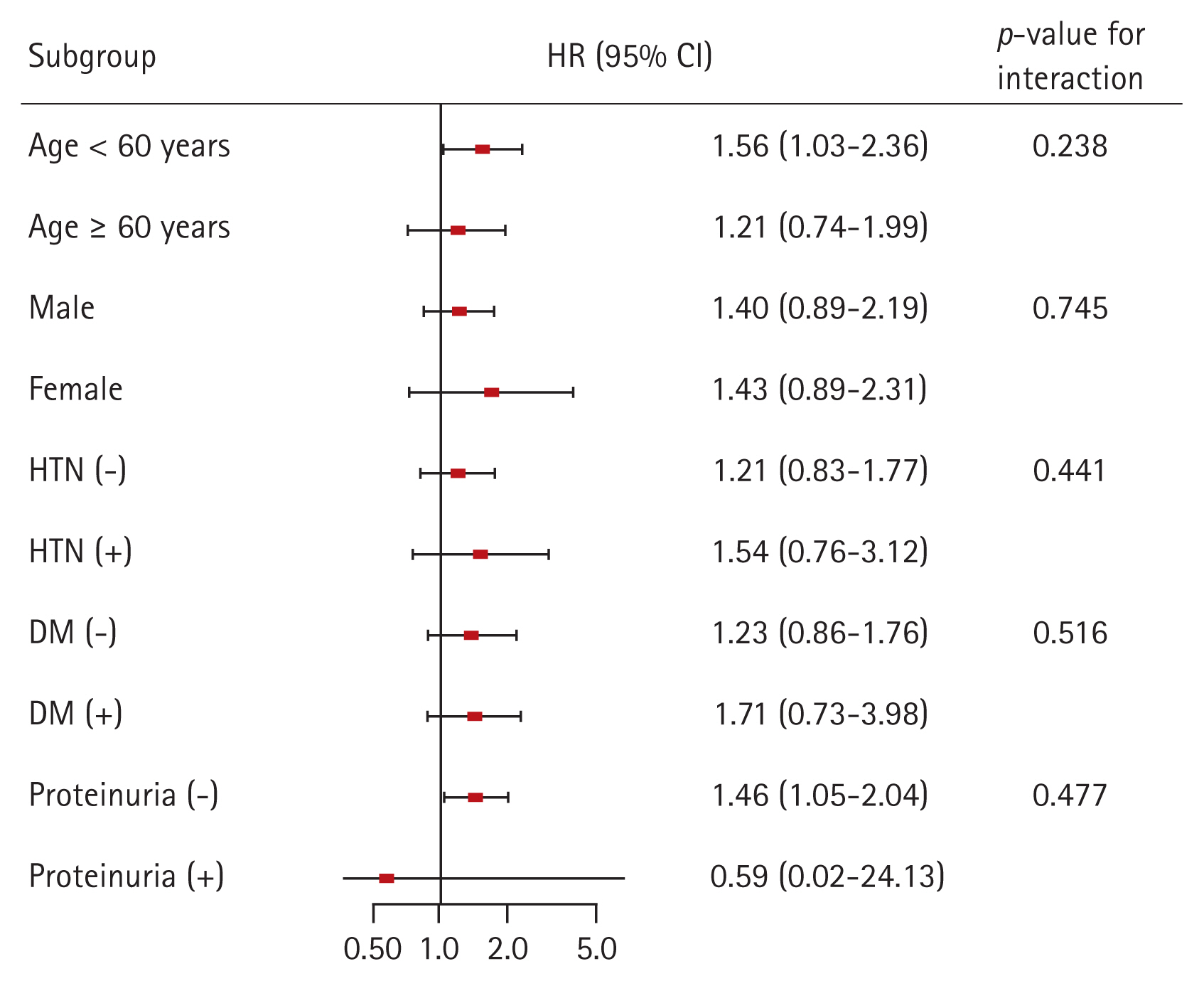
Finally, we conducted subgroup analyses to evaluate whether the association between obesity and the risk for incident CKD is modified by some factors (Fig. 6). We classified the degree of obesity according to the individualŌĆÖs WHR. The subgroups were stratified by age (< 60 or Ōēź 60 yr), sex, HTN, DM, and proteinuria. Multivariable Cox regression analysis revealed that p for interaction was > 0.05 for all subgroups, suggesting that the association of obesity with increased risk for incident CKD is not modified by these factors.
An additional subgroup analysis was performed to examine whether the effect of obesity reduction on the risk for incident CKD is modified by some factors (Supplementary Fig. 1). In the multivariable Cox regression analysis, p for interaction was > 0.05 for all subgroups, suggesting that the association of obesity reduction with decreased risk for incident CKD is not modified by these factors.
In this study, we found that obesity has an effect on CKD development in a population with normal renal function. Higher BMI and WHR significantly increased the risk for incident CKD in the multivariable analyses. Moreover, reducing obesity was beneficial for renal prognosis. These findings suggest that maintaining an appropriate weight is important for preserving renal function in a population with normal renal function. In addition, longitudinal changes in the metabolic profile showed that the obesity group had consistently poor metabolic profile. Moreover, our subgroup analyses suggest that obesity had an effect on development of CKD regardless of age, sex, HTN, and DM. This result reinforces the importance of obesity management in the general population.
Previous large surveys in a multiracial population documented that high BMI is strongly associated with the risk for CKD [8]. Another study showed that high BMI was a risk factor for CKD in Japanese men, but not in Japanese women [16]. Although BMI is the most useful index for obesity, it cannot distinguish between weight from muscle and fat, and between central and peripheral obesity. A previous study showed that WHR was more independently predictive of coronary heart disease than waist circumference or BMI in a general population cohort [17]. Elsayed et al. demonstrated that WHR but not BMI was a risk factor for incident CKD [18]. In the current study, both BMI and WHR were independent risk factors for incident CKD, but the magnitude of the effect of WHR was higher than that of BMI. BMI only provides body mass volume based on height, whereas WHR provides information about body shape and fat distribution, such as central obesity, which is the true meaning of obesity.
This study also showed that renal prognosis was better in individuals with reduced than in those with maintained obesity. Obesity is a well-known risk factor for cardiovascular disease, DM, stroke, and all-cause mortality [5,6,19,20]. HTN and DM are established risk factors for CKD, and a recent study showed that dyslipidemia is independently associated with incident CKD in the general population [21,22]. Obesity would affect renal function by causing these diseases. However, as mentioned in the studies above, obesity itself is a risk factor for CKD. The current study also revealed that obesity was independently associated with incident CKD after adjustment for HTN, DM, and LDL cholesterol. The mechanisms by which obesity directly affects renal function are unclear. One possible explanation is glomerular hyperfiltration/HTN. Bosma et al. [23] revealed that as BMI increases, effective renal plasma flow decreases and filtration fraction (the ratio of GFR and effective renal plasma flow) increases. The only way to explain GFR maintenance despite decreased renal plasma flow is glomerular hyperfiltration. Animal data also showed that obesity was associated with increased arterial pressure, glomerular hyperfiltration, and structural kidney damage, such as increased mesangial matrix and thickening of the glomerular and tubular basement membranes [24,25]. In fact, adipocytes produce a variety of factors that may affect renal microcirculation, such as angiotensinogen, leptin, and asymmetric dimethyl arginine, which is the most important endogenous inhibitor of nitric oxide synthase [9]. Adipocyte secretory products also directly stimulate aldosterone secretion that may be responsible for obesity-related HTN [26,27]. A previous study showed that weight reduction decreases plasma renin activity and aldosterone levels, and improves high blood pressure [28]. Although it has not yet been investigated whether these mechanisms observed in experiments also apply to healthy human, efforts to improve obesity, a modifiable factor, would be valuable for protecting renal function.
This study has several limitations. First, a causal relationship between obesity and CKD development could not fully be established due to the observational study design. Second, in the evaluation of the effect of obesity reduction, the sample size was small because only the obesity group at baseline was included. Further studies with larger sample sizes are needed.
However, our study also has certain strengths. Namely, both BMI and WHR were used to measure obesity and their influence on CKD development was compared. In addition, to assess the significance of obesity reduction, we categorized obesity reduction using the baseline and 4-year WHR. With these strengths, this study demonstrated that obesity, particularly the group with high WHR, was an independent risk factor for CKD development, suggesting that reducing obesity is beneficial for renal prognosis.
In conclusion, High BMI and high WHR were associated with incident CKD. Efforts to reduce fat tissue and obesity, such as weight loss, exercise, and diet control, can protect renal function in the healthy population.
1. Higher BMI and WHR significantly increased the risk for incident CKD, and the magnitude of the effect of WHR was higher than that of BMI.
2. Renal prognosis was better in individuals with reduced obesity than in those with maintained obesity.
3. Obesity has an effect on CKD development in a population with normal renal function, and this finding suggest that maintaining an appropriate weight is important for preserving renal function.
Acknowledgments
The data used in this study were obtained from the Korean Genome and Epidemiology Study (KoGES; 6635-302), National Institute of Health, Korea Disease Control and Prevention Agency, Republic of Korea. The datasets analyzed in this study are available from the corresponding author on reasonable request. We thank Dr Jinseob Kim and Youngho Kim for providing technical support in data analysis.
Notes
CRedit authorship contributions
Su Hyun Song: conceptualization, methodology, formal analysis, writing - original draft; Tae Ryom Oh: methodology, investigation, data curation, validation, software; Sang Heon Suh: formal analysis, validation, software, visualization; Hong Sang Choi: methodology, investigation, data curation, validation, software, writing - original draft; Chang Seong Kim: validation, software, visualization; Seong Kwon Ma: writing - review & editing, visualization, supervision; Soo Wan Kim: resources, supervision, funding acquisition; Eun Hui Bae: conceptualization, writing - review & editing, supervision, project administration, funding acquisition
Funding
This research was funded by Chonnam National University Hospital Biomedical Research Institute Grant (BCRI 23014&24032). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2019R1A2C1003971&RS-2023-00217317).
Figure┬Ā1
Study flow diagram. KoGES, Korean Genome and Epidemiology Study; eGFR, estimated glomerular filtration rate.
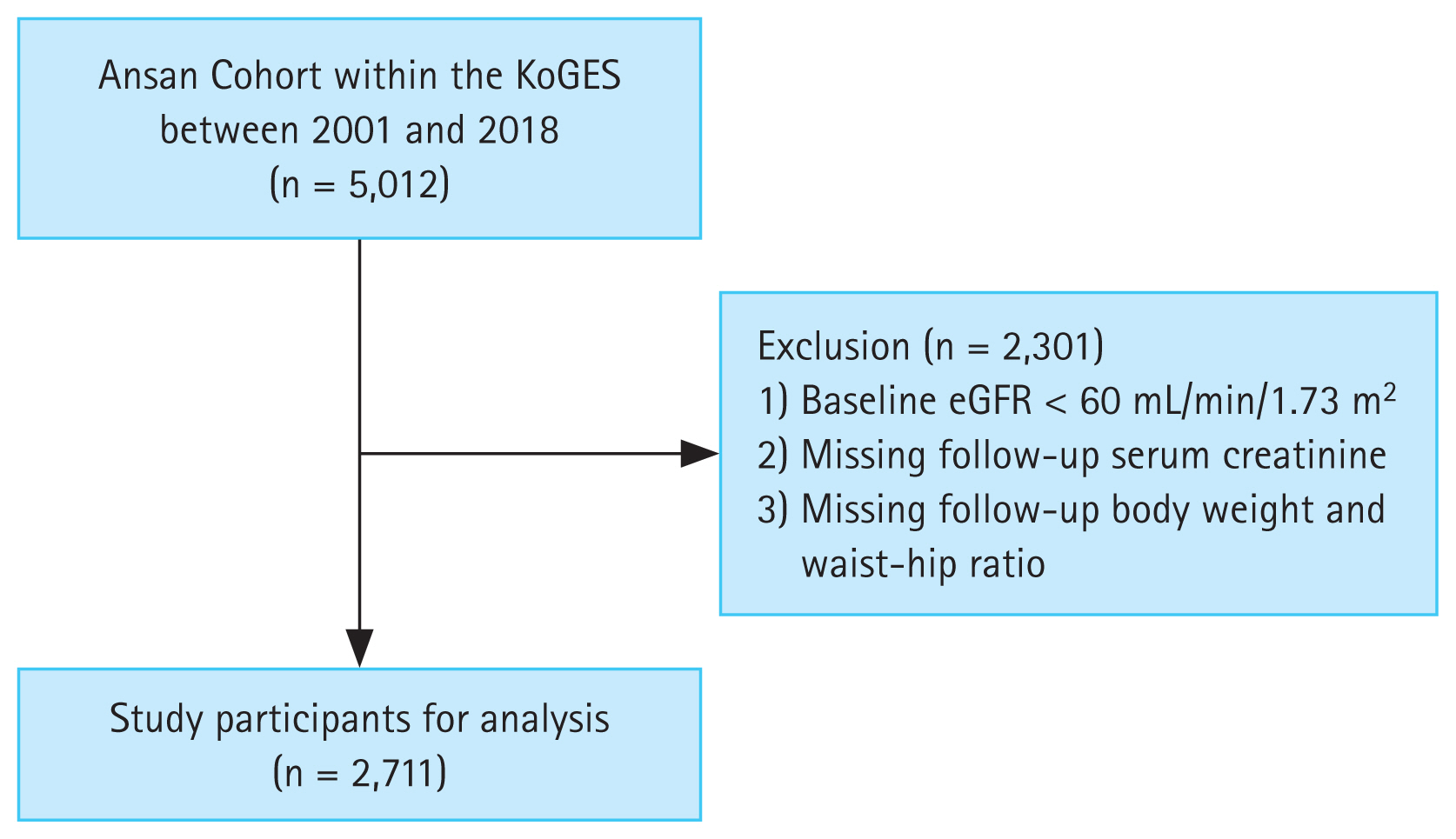
Figure┬Ā2
Longitudinal changes in metabolic profile parameters during the follow-up period. The X-axis denotes follow-up duration in years. BMI, body mass index; HbA1c, hemoglobin A1c; HOMA-IR, homeostasis model assessment-insulin resistance; CRP, C-reactive protein; HDL, high-density lipoprotein.
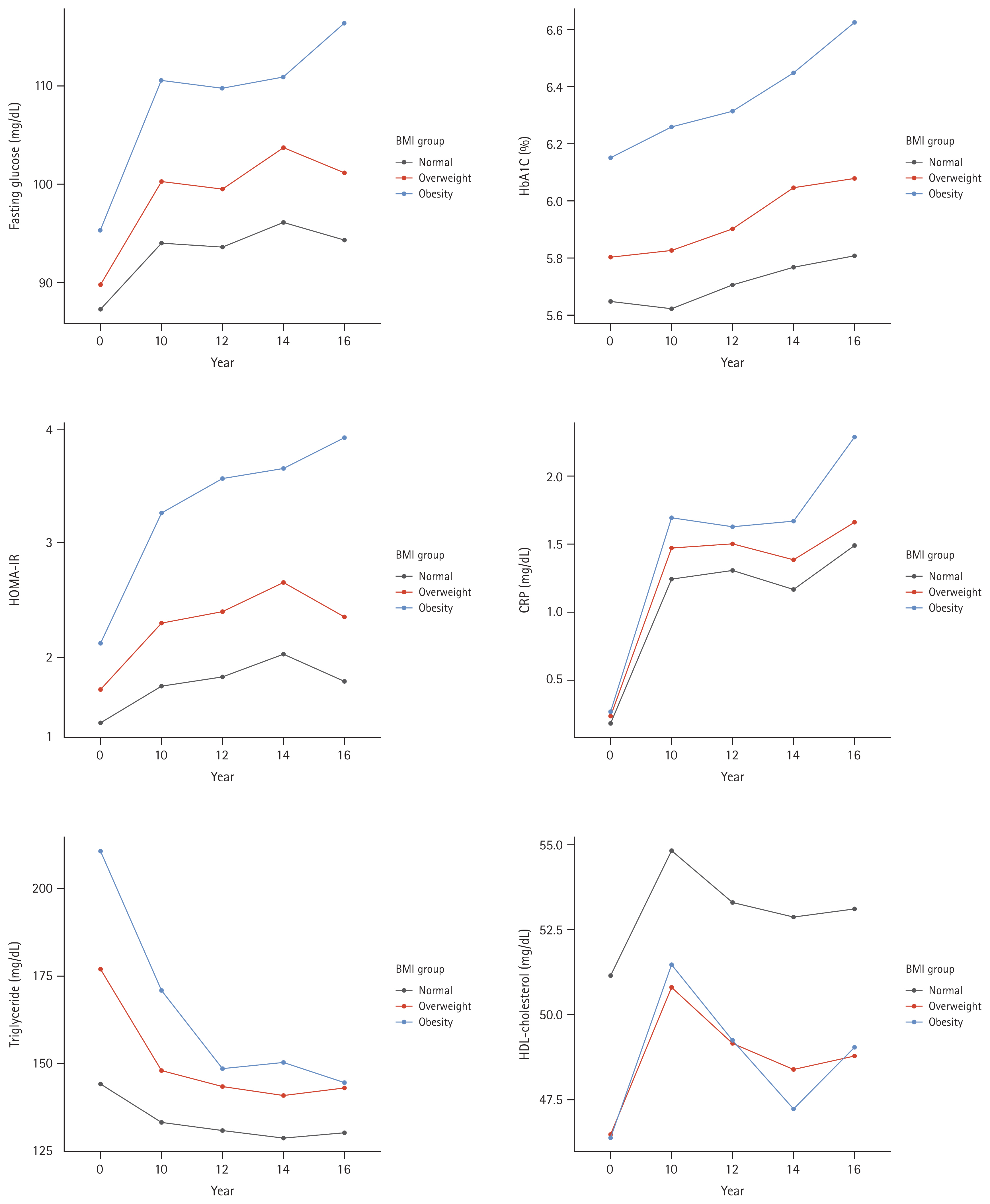
Figure┬Ā3
Log-transformed adjusted hazard ratio and 95% confidence interval for incident CKD probability associated with (A) BMI and (B) WHR. The BMI and WHR exhibited a positive correlation with the risk for incident CKD. CKD, chronic kidney disease; BMI, body mass index; WHR, waist-to-hip ratio.
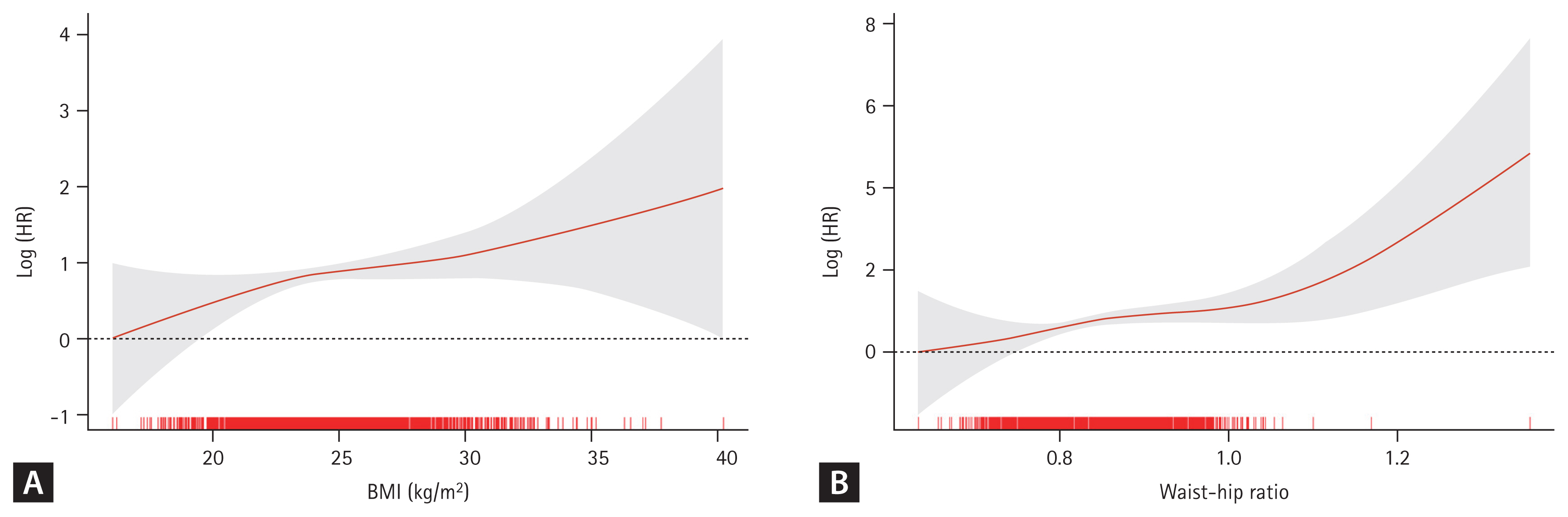
Figure┬Ā4
KaplanŌĆōMeier free-of-CKD probability curve with log-rank test between the obesity group and incident CKD. Obesity according to the (A) BMI and (B) WHR was associated with poor free-of-CKD probability. The X-axis denotes time-to events duration in days. CKD, chronic kidney disease; BMI, body mass index; WHR, waist-to-hip ratio.
Figure┬Ā5
KaplanŌĆōMeier free-of-CKD probability curve with log-rank test according to obesity reduction. The X-axis denotes time-to events duration in days. CKD, chronic kidney disease; WHR, waist-to-hip ratio.
Figure┬Ā6
Multivariable Cox regression analysis for incident CKD according to WHR, stratified by subgroups. Models were adjusted for age, sex, HTN, DM, alcohol consumption, smoking, LDL cholesterol, hemoglobin, CRP, and baseline eGFR. HR, hazard ratio; CI, confidence interval; HTN, hypertension; DM, diabetes mellitus; CKD, chronic kidney disease; WHR, waist-to-hip ratio; LDL, low-density lipoprotein; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate.
Table┬Ā1
Baseline characteristics of the total study population according to BMI
| Variable | Total population (n = 2,712) | p value | ||
|---|---|---|---|---|
| Normal (n = 1,542) | Overweight (n = 1,045) | Obesity (n = 125) | ||
| Age (yr) | 48.2 ┬▒ 7.4 | 49.3 ┬▒ 7.5 | 50.2 ┬▒ 8.2 | < 0.001 |
| Male, sex | 778 (50.5) | 573 (54.8) | 42 (33.6) | < 0.001 |
| Education | < 0.001 | |||
| ŌĆāNo education or elementary school graduate | 180 (11.7) | 166 (15.9) | 33 (26.4) | |
| ŌĆāMiddle school graduate | 339 (22.0) | 224 (21.4) | 27 (21.6) | |
| ŌĆāHigh school graduate | 715 (46.4) | 428 (41.0) | 45 (36.0) | |
| ŌĆāMore than college graduate | 308 (19.9) | 227 (21.7) | 20 (16.0) | |
| Alcohol drinking status | 0.022 | |||
| ŌĆāNever | 687 (44.6) | 414 (39.6) | 64 (51.2) | |
| ŌĆāFormer drinker | 85 (5.5) | 54 (5.2) | 8 (6.4) | |
| ŌĆāDrinker | 770 (49.9) | 577 (55.2) | 53 (42.4) | |
| Smoking status | < 0.001 | |||
| ŌĆāNever | 910 (59.0) | 575 (55.0) | 92 (73.6) | |
| ŌĆāFormer smoker | 276 (17.9) | 225 (21.5) | 14 (11.2) | |
| ŌĆāCurrent smoker | 356 (23.1) | 245 (23.5) | 19 (15.2) | |
| Household income/mo, in won (more than 2,000 USD)a) | 867 (56.2) | 597 (57.1) | 61 (48.8) | 0.028 |
| Diabetes mellitus | 78 (5.1) | 62 (5.9) | 17 (13.6) | < 0.001 |
| Hypertension | 108 (7.0) | 177 (16.9) | 33 (26.4) | < 0.001 |
| Systolic BP (mmHg) | 114.3 ┬▒ 16.8 | 120.7 ┬▒ 16.1 | 125.0 ┬▒ 16.8 | < 0.001 |
| Diastolic BP (mmHg) | 76.4 ┬▒ 11.3 | 80.8 ┬▒ 11.3 | 84.5 ┬▒ 11.6 | < 0.001 |
| BUN (mg/dL) | 14.0 ┬▒ 3.3 | 14.6 ┬▒ 3.6 | 14.7 ┬▒ 3.2 | < 0.001 |
| Creatinine (mg/dL) | 0.856 ┬▒ 0.2 | 0.893 ┬▒ 0.2 | 0.851 ┬▒ 0.2 | < 0.001 |
| eGFR (mL/min/1.73 m2) | 94.7 ┬▒ 15.3 | 91.5 ┬▒ 16.1 | 90.9 ┬▒ 15.7 | < 0.001 |
| Hemoglobin (g/dL) | 13.5 ┬▒ 1.6 | 14.0 ┬▒ 1.6 | 13.6 ┬▒ 1.4 | < 0.001 |
| HbA1c (%) | 5.7 ┬▒ 0.9 | 5.8 ┬▒ 0.9 | 6.2 ┬▒ 1.2 | < 0.001 |
| Na (mmol/L) | 142.7 ┬▒ 2.2 | 142.9 ┬▒ 2.2 | 142.4 ┬▒ 2.2 | 0.007 |
| K (mmol/L) | 4.5 ┬▒ 0.4 | 4.4 ┬▒ 0.5 | 4.4 ┬▒ 0.5 | 0.584 |
| CRP (mg/dL) | 0.2 ┬▒ 0.4 | 0.2 ┬▒ 0.4 | 0.3 ┬▒ 0.3 | < 0.001 |
| HDL cholesterol (mg/dL) | 51.1 ┬▒ 12.0 | 46.5 ┬▒ 10.3 | 46.4 ┬▒ 10.5 | < 0.001 |
| Triglyceride (mg/dL) | 144.3 ┬▒ 98.3 | 177.0 ┬▒ 101.2 | 210.8 ┬▒ 160.5 | < 0.001 |
| LDL cholesterol (mg/dL) | 117.2 ┬▒ 32.1 | 122.3 ┬▒ 33.1 | 118.8 ┬▒ 39.3 | 0.001 |
Table┬Ā2
HR for incident chronic kidney disease by BMI and WHR with Cox proportion hazard models
REFERENCES
1. Boutari C, Mantzoros CS. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022;133:155217.



2. Adler NE, Prather AA. Risk for type 2 diabetes mellitus: person, place, and precision prevention. JAMA Intern Med 2015;175:1321ŌĆō1322.


4. Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group. The metabolic syndrome--a new world-wide definition. Lancet 2005;366:1059ŌĆō1062.


5. Katta N, Loethen T, Lavie CJ, Alpert MA. Obesity and coronary heart disease: epidemiology, pathology, and coronary artery imaging. Curr Probl Cardiol 2021;46:100655.


6. Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 2006;355:763ŌĆō778.


7. Foster MC, Hwang SJ, Larson MG, et al. Overweight, obesity, and the development of stage 3 CKD: the Framingham Heart Study. Am J Kidney Dis 2008;52:39ŌĆō48.



8. Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med 2006;144:21ŌĆō28.


9. Mallamaci F, Tripepi G. Obesity and CKD progression: hard facts on fat CKD patients. Nephrol Dial Transplant 2013;28(Suppl 4):iv105ŌĆōiv108.


10. Wickman C, Kramer H. Obesity and kidney disease: potential mechanisms. Semin Nephrol 2013;33:14ŌĆō22.


11. Liyanage T, Toyama T, Hockham C, et al. Prevalence of chronic kidney disease in Asia: a systematic review and analysis. BMJ Glob Health 2022;7:e007525.



12. World Health Organization. Obesity and overweight [Internet] Geneva: World Health Organization, 2021. [cited 2023 Aug 8]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
13. Kim Y, Han BG, KoGES group. Cohort profile: the Korean Genome and Epidemiology Study (KoGES) consortium. Int J Epidemiol 2017;46:e20.


14. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604ŌĆō612.



15. Haam JH, Kim BT, Kim EM, et al. Diagnosis of obesity: 2022 update of clinical practice guidelines for obesity by the Korean Society for the Study of Obesity. J Obes Metab Syndr 2023;32:121ŌĆō129.



16. Andreacchi AT, Griffith LE, Guindon GE, et al. Body mass index, waist circumference, waist-to-hip ratio, and body fat in relation to health care use in the Canadian Longitudinal Study on Aging. Int J Obes (Lond) 2021;45:666ŌĆō676.



17. Iseki K, Ikemiya Y, Kinjo K, Inoue T, Iseki C, Takishita S. Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int 2004;65:1870ŌĆō1876.


18. Canoy D, Boekholdt SM, Wareham N, et al. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population-based prospective study. Circulation 2007;116:2933ŌĆō2943.


19. Elsayed EF, Sarnak MJ, Tighiouart H, et al. Waist-to-hip ratio, body mass index, and subsequent kidney disease and death. Am J Kidney Dis 2008;52:29ŌĆō38.



20. Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes 2014;7:587ŌĆō591.


21. Horn JW, Feng T, M├Ėrkedal B, et al. Obesity and risk for first ischemic stroke depends on metabolic syndrome: the HUNT study. Stroke 2021;52:3555ŌĆō3561.


22. Liang X, Ye M, Tao M, et al. The association between dyslipidemia and the incidence of chronic kidney disease in the general Zhejiang population: a retrospective study. BMC Nephrol 2020;21:252.




23. Bosma RJ, van der Heide JJ, Oosterop EJ, de Jong PE, Navis G. Body mass index is associated with altered renal hemodynamics in non-obese healthy subjects. Kidney Int 2004;65:259ŌĆō265.


24. OŌĆÖDonnell MP, Kasiske BL, Cleary MP, Keane WF. Effects of genetic obesity on renal structure and function in the Zucker rat. II. Micropuncture studies. J Lab Clin Med 1985;106:605ŌĆō610.

25. Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol 2001;12:1211ŌĆō1217.


26. Ehrhart-Bornstein M, Arakelyan K, Krug AW, Scherbaum WA, Bornstein SR. Fat cells may be the obesity-hypertension link: human adipogenic factors stimulate aldosterone secretion from adrenocortical cells. Endocr Res 2004;30:865ŌĆō870.


- TOOLS
-
METRICS

- Related articles
-
The role of roxadustat in chronic kidney disease patients complicated with anemia2023 March;38(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement figure 1
Supplement figure 1 Print
Print


