INTRODUCTION
Thromboembolic risk after cardioversion of atrial fibrillation, either pharmacological or electrical, has been known to be associated with atrial mechanical dysfunction (stunning) caused by rapid heart rate per se or direct effect of DC energy. Recently, it has been shown that patients with chronic atrial flutter also have a significant, albeit low, risk of embolic events after restoration of sinus rhythm1–3).
Although electrical cardioversion or radiofrequency catheter ablation of atrial flutter are common procedures, atrial stunning after restoration of sinus rhythm has not been investigated systematically. The aim of this study was to determine whether the atrial mechanical dysfunction was present after successful RF ablation of chronic atrial flutter and whether this change was reversible.
MATERIALS AND METHODS
1. Study patients
Patients with persistent atrial flutter of 33.0 (33.5 months in duration were included in this study. During follow-up, patients with documented or having symptoms suggesting paroxysmal atrial fibrillation were excluded. Atrial flutter was successfully terminated during RF ablation and normal sinus rhythm was maintained throughout the study duration. Atrial flutter recurred in three patients following RF ablation. They were enrolled in the study after second RF ablation. In those cases, second RF ablation was performed in 1 month, 1.5 months and 3 months after recurrence of AFL, respectively.
2. Study protocol and RF ablation
The study protocol was approved by the Ethics Committee of Ulsan University Medical College. All patients gave written informed consent for the ablation procedure and study protocol. Electrophysiologic tests were performed after discontinuation of all antiarrhythmic agents for five half-lives. In some patients, Transesophageal echocardiography (TEE) was performed to exclude the presence of atrial thrombi. Midazolam and fentanyl were used for sedation, if necessary. Catheters were placed in the high lateral right atrium (RA), coronary sinus (CS), His bundle recording position. A Duo-decapolar mapping catheter (Daig Livewire, U.S.A) was placed around the interatrial septum, tricuspid valve (TV) annulus and right atrial free wall. The RF ablation of AFL was performed as described previously4,5).
Successful ablation was defined as bidirectional isthmic conduction block. Conduction block was demonstrated by pacing either from the coronary sinus or low lateral RA. DC shock or antiarrhythmic agents were not used during the procedure.
3. Echocardiographic analysis
Transthoracic 2-D and pulsed wave (PW) doppler echocardiography were performed with Sonos 5500 of Hewlett Packard. Transmitral flow velocities - peak E and A waves - were recorded at the tip of mitral valve leaflets in apical 4 chamber view. Mitral annular velocities with doppler tissue imaging technique (DTI) - annular E and A waves - were recorded with PW Doppler (range from −30 to 30 cm/sec). For this purpose, 5 mm-sample volume was placed at the lateral corner of the mitral annulus in apical 4 chamber view.
4. Follow-up
After successful RF ablation, intravenous heparin was administered to keep target activated partial thromboplastin time (aPTT) of 60–80 seconds, which was later switched to warfarin. Patients were discharged without antiarrhythmic medication. Echocardiographic evaluation was performed at 1 day, 1 month, 3 months, 6–12 months after successful ablation. All patients were coumadinized to maintain INR 2–3 for more than 3 weeks.
5. Statistics
All values are reported as mean (SD unless noted otherwise). Wilcoxon signed rank test was used to compare changes in measurements of atrial function and dimension before and after RF ablation. Each statistical value was adjusted by Bonferroni method. Analysis with p values < 0.05 were considered significant.
RESULTS
1. Clinical characteristics of the study patients
The subjects of this study were 13 patients (12 men and 1 woman, mean age ± SD 56.7 ± 14.4 years). All patients had documented type I AFL, defined electrocardiographically as inverted P waves inscribing saw-tooth pattern in inferior leads (lead II, III, aVF), and upright P waves in lead V1. The duration of documented AFL was 33.0 ± 33.5 months, as determined by the time from initial 12-lead ECG diagnosis to the time of the RF ablation. Two patients had hypertension, two had dilated cardiomyopathy, one had an ASD closed with patch previously, one had COPD and the remaining seven had no structural heart disease. Clinical characteristics of the study patients are presented in Table 1.
2. Radiofrequency ablation
The mean AFL cycle length of the patients were 285.2 ± 41.6 msec. Counterclockwise activation sequence in the right atrium was confirmed in 11 patients, and clockwise activation in 2 patients. Anatomically guided linear RF ablation of the TV-IVC isthmus was performed during AFL. All counterclockwise AFLs were successfully ablated by TV-IVC isthmus ablation. Bidirectional conduction block was verified in all patients. In the two patients with clockwise AFL, one was terminated by TV-IVC linear ablation but bidirectional conduction block was achieved after addition of TV-CS lesion. In the other with clockwise type AFL, TV-IVC lesion was ineffective for termination. AFL termination and bidirectional conduction block were achieved only after TV-CS lesion. Atrial flutter recurred in three patients in 3 days, 4 months, and 2.5 years after the initial successful ablation. The second ablation procedures were performed in all with success. In those cases, data for the study were obtained after the second RF ablation. Fluoroscopic time for the procedure was 49.4 ± 18.6 min, and the number of RF applications were 32.0±16.7.
3. Atrial mechanical function assessed by transmitral doppler flow velocity
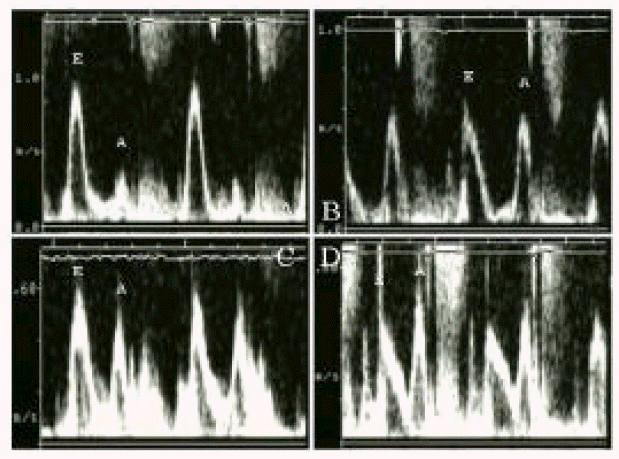
Atrial mechanical function was assessed by the serial measurement of the mitral inflow A wave velocity and mitral annular motion. Mean mitral inflow A wave velocity measured on post-ablation day. 1 was 40 (18.4 cm/sec. It was increased to 56.7 ± 19.9 cm/sec after 1 month of ablation (p<0.05 vs. post-ablation day 1), but did not show further increase thereafter (55.7 ± 21.5 cm/sec after 3 months, 64.6 ± 19.1 cm/sec after 6–12 months, p = ns vs. post-ablation 1 month). These results are presented in Table 2, Figure 1 and Figure 3A.
4. Atrial mechanical function assessed by mitral annular motion
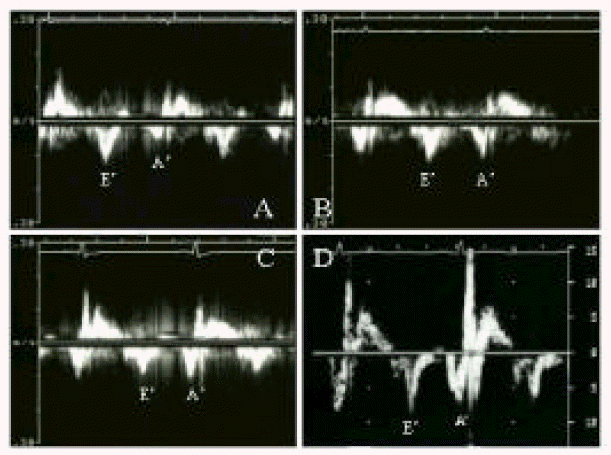
Mitral annular velocity was measured in 9 patients (Table 3, Figure 2). It was decreased at post-ablation day 1, but increased significantly after 1 month of ablation (4.7 ± 1.5 cm/sec vs. 10.3 ± 2.9 cm/sec, p<0.05). After then, no statistically significant improvements were noted. The serial change in mitral inflow A wave velocity and annular motion was compared in Table 3, Figure 2 and Figure 3B.
5. Change in left atrial dimension
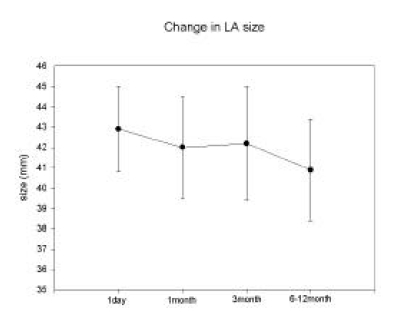
Left atrial dimensions, measured with 2D echocardiography in parasternal long axis view, at 1 day, 1 month, 3 months and 6–12 months after ablation were 42.9 ± 7.1, 42.0 ± 7.8, 42.2 ± 8.5, and 40.9 ± 7.5 mm, respectively. These values were not significantly different. This serial change in LA dimension is depicted in Figure 4. The duration of flutter and LA size were not correlated in this study.
DISCUSSION
1. Main findings
This study demonstrates that (1) atrial stunning occurs after termination of AFL by RF ablation, (2) left atrial mechanical function recovers in one month after restoration of normal sinus rhythm and (3) this recovery of mechanical function is not associated with decrease in the left atrial size.
It has long been believed that patients with chronic AFL have lower thromboembolic risk and anticoagulation was not routinely recommended during or after cardioversion13). This belief derived from the inference that the atrial in chronic atrial flutter have more synchronous activity compared with those of chronic atrial fibrillation. However, recent evidence demonstrates that patients with chronic atrial flutter show considerable left atrial and/or left atrial appendageal stunning and formation of new or aggravation of spontaneous echo contrast after catheter ablation6,7). Serial echocardiographic follow-up demonstrated significant improvement of atrial mechanical dysfunction over 3–6 weeks after RF ablation9).
Transesophageal echocardiography, performed immediately after successful catheter ablation of atrial flutter, showed atrial mechanical dysfunction despite the presence of sinus rhythm. The atrial mechanical dysfunction may be the cause of increased thromboembolic risk3,8) in this population.
This study demonstrated long-term change in atrial mechanical function assessed by TTE and DTI technique. Post-RF atrial mechanical dysfunction after restoration of normal sinus rhythm was recovered within one month and reached a plateau over one year. We also used doppler tissue imaging technique to verify this pattern of recovery avoiding load-dependent alteration of the transmitral flow velocity which showed similar results.
2. Mechanism of atrial stunning
The mechanism of atrial stunning after DC cardioversion of atrial flutter or fibrillation is poorly understood. Since the patients of this study did not receive electrical shock or pharmacologic treatment, we assumed that atrial dysfunction would be a direct result of rapid atrial rate. This result is compatible with other previous reports on the mechanism of atrial stunning9,10).
3. Importance of cardioversion of AFL
Recently, Chen et al showed that atrial tachycardia11) causes atrial electrical remodeling, which is proportional to the rate of atrial excitation. Similarly, Franz showed that atrial flutter12) causes atrial electrical remodeling to a similar degree of chronic atrial fibrillation. Electrical remodeling in AFL reduces the duration of atrial effective refractoriness, predisposing transformation of atrial flutter to atrial fibrillation. It means that atrial flutter has the potential to progress to atrial fibrillation. In our study, the percent recovery of atrial contraction showed the tendency of inverse correlation to the duration of atrial flutter. It suggests that restoration of mechanical dysfunction becomes more difficult as the duration of AFL becomes longer. Taken together with Franz’s observation, sinus rhythm in patients with AFL should be restored aggressively before its progression to electrical or mechanical remodeling. The fact that the atrial chamber size was not reduced over a one year follow-up period, may suggest that atrial geometrical change has progressed to an irreversible stage already, or geometrical change lags in the recovery time course compared with that of contractile function. However, it cannot be ruled out that atrial geometrical change preceded atrial flutter, and served as a substrate for the development of atrial flutter. This egg and hen debate cannot be answered from this study, but if we could demonstrate the restoration of atrial dimension in patients with shorter duration of AFL, it would be another reason to restore normal sinus rhythm in patients with AFL as early as possible.
4. Duration of anticoagulation
Although the need for routine anticoagulation in AFL is beyond dispute, incidences of thromboembolic events after cardioversion of AFL have been reported diversely13,14). However, definite presence of atrial stunning, newly developed or aggravated spontaneous echo contrast after cardioversion, implies that atrial flutter should be treated as AF at least after cardioversion. Marked impairment of atrial mechanical function and restoration of it with time after AFL ablation in our study was also in accordance with findings of recent reports7,9).
The optimal duration of anticoagulation after cardioversion of atrial flutter has not been established. Our study revealed that atrial contractile function significantly improved one month after restoration of sinus rhythm. It suggests that routine anticoagulation may not be necessary after this period. In a recent paper by Sparks et al.9), appendageal flow pattern or ejection fraction measured by transesophageal echocardiography showed that atrial function progressively improved over 3 weeks. This time frame of recovery is consistent with our results and suggests the need of anticoagulation for more than this period.
5. Study Limitation
Although we have shown the significant recovery of atrial contractile function after one month of ablation, we should have performed serial follow-up of appendageal flow with TEE to guide the duration of anticoagulation. But it was not feasible to do frequent follow-up TEE examination. Therefore, the recovery time course of atrial appendageal mechanical function could not be determined.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





