 |
 |
| Korean J Intern Med > Volume 39(3); 2024 > Article |
|
Abstract
Background/Aims
Methods
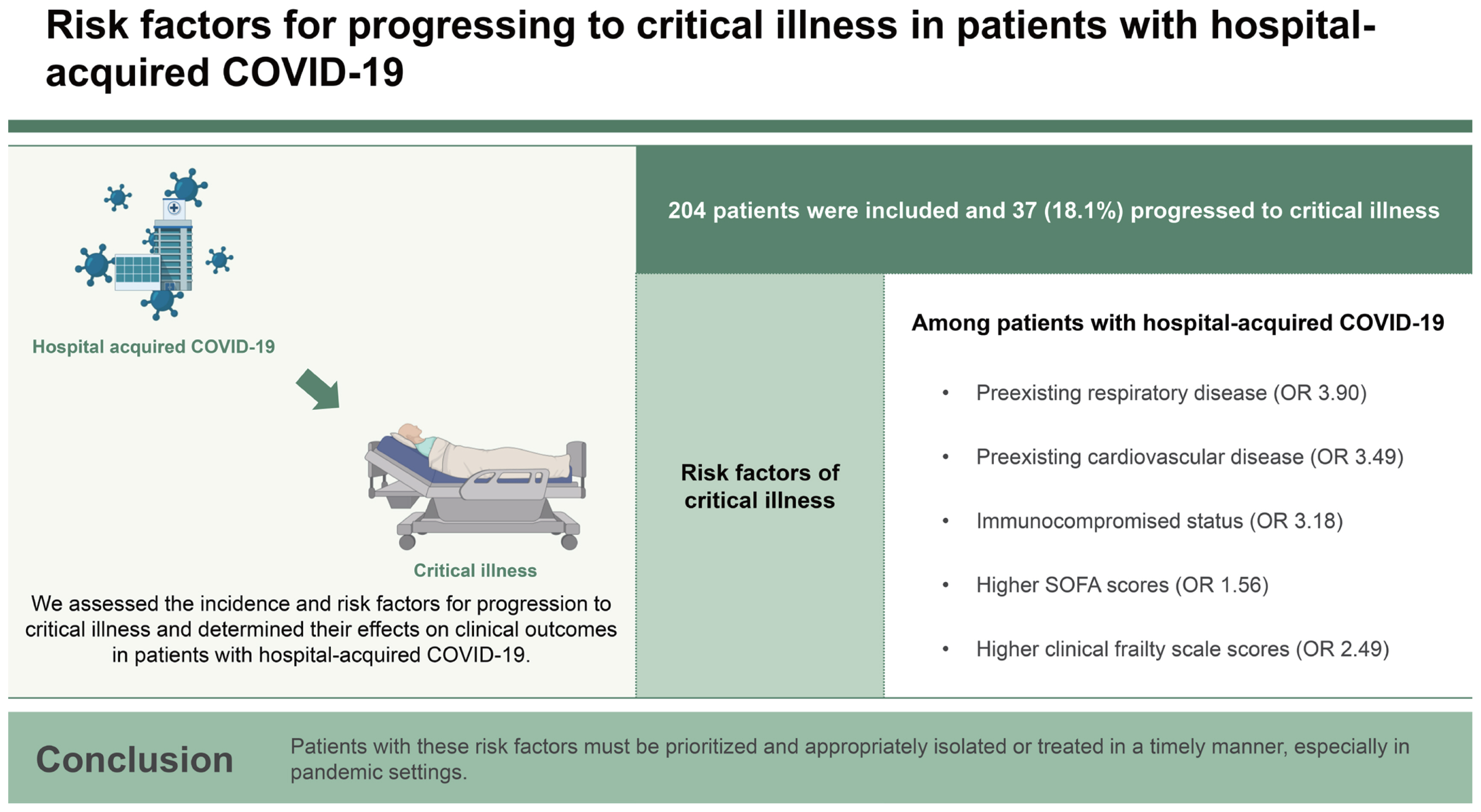
Results
Conclusions
Notes
Availability of data and materials
Figure 1.

Figure 2.
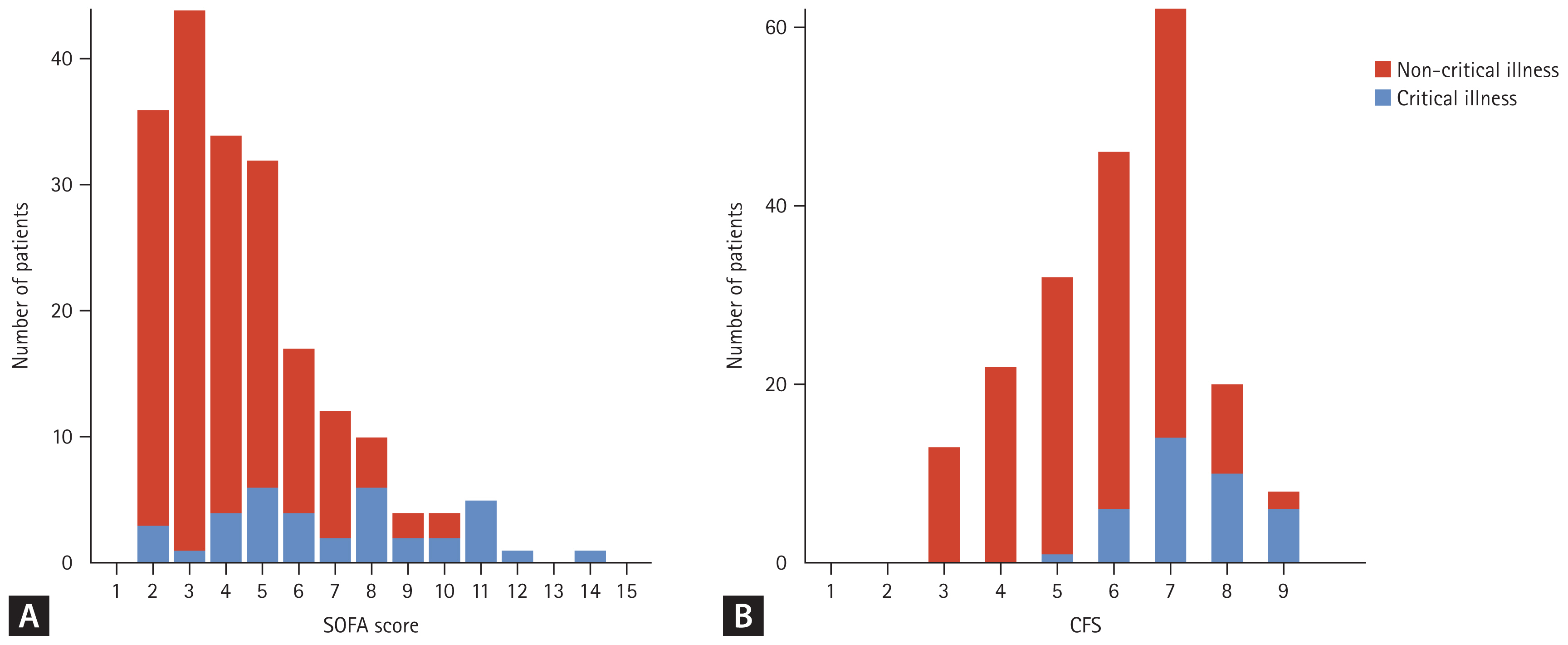
Table 1
| Variable | Total (n = 204) | Non-critical illness (n = 167) | Critical illness (n = 37) | p value |
|---|---|---|---|---|
| Age (yr) | 65 (53–74) | 65 (52–73) | 65 (55–77) | 0.452 |
| Sex, male | 127 (62.3) | 101 (60.5) | 26 (70.3) | 0.355 |
| Body mass index (kg/m2) | 22 (19–25) | 22 (20–25) | 19 (17–24) | 0.015 |
| Comorbidity | ||||
| Respiratory disease | 24 (11.8) | 15 (9.0) | 9 (24.3) | 0.019 |
| Cardiovascular disease | 45 (22.1) | 34 (20.4) | 11 (29.7) | 0.306 |
| Hypertension | 82 (40.2) | 69 (41.3) | 13 (35.1) | 0.611 |
| Neurologic disease | 67 (32.8) | 60 (35.9) | 7 (18.9) | 0.072 |
| Diabetes | 65 (31.9) | 56 (33.5) | 9 (24.3) | 0.372 |
| Chronic liver disease | 18 (8.8) | 12 (7.2) | 6 (16.2) | 0.152 |
| Chronic kidney disease | 35 (17.2) | 24 (14.4) | 11 (29.7) | 0.045 |
| Connective tissue disease | 21 (10.3) | 17 (10.2) | 4 (10.8) | > 0.999 |
| Solid malignancy | 60 (29.4) | 46 (27.5) | 14 (37.8) | 0.297 |
| Hematologic malignancy | 25 (12.3) | 19 (11.4) | 6 (16.2) | 0.593 |
| Immunocompromised | 84 (41.2) | 63 (37.7) | 21 (56.8) | 0.052 |
| Respiratory support at baseline | < 0.001 | |||
| No oxygen support | 155 (76.0) | 141 (84.4) | 14 (37.8) | |
| Nasal prong | 36 (17.6) | 26 (15.6) | 10 (27.0) | |
| High-flow nasal cannula | 13 (6.4) | 0 (0) | 13 (35.1) | |
| From hospitalization to COVID-19 diagnosis (d) | 16 (9–27) | 15 (9–25) | 24 (16–37) | 0.001 |
| Sequential organ failure assessment scorea) | 4 (3–6) | 4 (3–5) | 7 (5–9) | < 0.001 |
| Clinical frailty scaleb) | 6 (5–7) | 6 (5–7) | 7 (7–8) | < 0.001 |
| Former antibiotics | 145 (71.1) | 113 (67.7) | 32 (86.5) | 0.037 |
| Critical illness | ||||
| Acute respiratory distress syndrome | 10 (4.9) | 10 (27.0) | ||
| Septic shock | 22 (10.8) | 22 (59.5) | ||
| Need for life-sustaining therapy | 37 (18.1) | 37 (100.0) | ||
| Hospital length of stay (d)c) | 11 (7–24) | 10 (7–20) | 22 (8–41) | 0.002 |
| Discharge statusd) | 0.804 | |||
| Home | 136/179 (76.0) | 124/162 (76.5) | 12/17 (70.6) | |
| Transfer to other hospital or nursing home | 43/179 (24.0) | 38/162 (23.5) | 5/17 (29.4) | |
| In-hospital mortality | 25 (12.3) | 5 (3.0) | 20 (54.1) | < 0.001 |
| 28-day mortality | 17 (8.3) | 2 (1.2) | 15 (40.5) | < 0.001 |
Table 2
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | p value | OR (95% CI)a) | p value | |
| Age | 1.01 (0.99–1.03) | 0.471 | ||
|
|
||||
| Sex, male | 0.65 (0.30–1.40) | 0.269 | ||
|
|
||||
| Body mass index | 0.91 (0.83–0.99) | 0.035 | ||
|
|
||||
| Comorbidity | ||||
|
|
||||
| Respiratory disease | 3.26 (1.30–8.17) | 0.012 | 3.90 (1.04–15.18) | 0.044 |
|
|
||||
| Cardiovascular disease | 1.65 (0.74–3.68) | 0.217 | 3.49 (1.11–11.27) | 0.032 |
|
|
||||
| Hypertension | 0.77 (0.37–1.62) | 0.488 | ||
|
|
||||
| Diabetes | 0.64 (0.28–1.44) | 0.279 | 0.41 (0.13–1.18) | 0.112 |
|
|
||||
| Chronic liver disease | 2.29 (0.81–6.50) | 0.118 | ||
|
|
||||
| Chronic kidney disease | 2.52 (1.10–5.76) | 0.028 | ||
|
|
||||
| Connective tissue disease | 1.07 (0.34–3.39) | 0.909 | ||
|
|
||||
| Solid malignancy | 1.60 (0.76–3.38) | 0.216 | ||
|
|
||||
| Hematologic malignancy | 1.51 (0.56–4.08) | 0.419 | ||
|
|
||||
| Immunocompromised | 2.17 (1.05–4.46) | 0.036 | 3.18 (1.11–9.16) | 0.025 |
|
|
||||
| Abnormal white blood cell countb) | 3.38 (1.60–7.12) | 0.001 | ||
|
|
||||
| Abnormal heart ratec) | 2.76 (1.33–5.72) | 0.006 | ||
|
|
||||
| Abnormal body temperatured) | 1.08 (0.48–2.19) | 0.953 | ||
|
|
||||
| SOFA score | 1.66 (1.40–1.98) | < 0.001 | 1.56 (1.28–1.96) | < 0.001 |
|
|
||||
| CFS | 2.99 (1.98–4.51) | < 0.001 | 2.49 (1.62–4.13) | < 0.001 |
|
|
||||
| Dexamethasone usee) | 2.29 (0.81–6.50) | 0.118 | ||
|
|
||||
| Remdesivir usee) | 1.06 (0.52–2.15) | 0.882 | ||
|
|
||||
| Former antibiotics | 3.06 (1.13–8.29) | 0.028 | ||
COVID-19, coronavirus disease 2019; OR, odds ratio; CI, confidence interval; SOFA, sequential organ failure assessment; CFS, clinical frailty scale.
a) Bidirectional stepwise selection for age, sex, body mass index, respiratory disease, cardiovascular disease, hypertension, diabetes, chronic liver disease, chronic kidney disease, connective tissue disease, solid malignancy, hematologic malignancy, immunocompromised status, white blood cell count, heart rate, body temperature, SOFA score, CFS, the use of dexamethasone, remdesivir before progression to critical illness, and former antibiotics use was conducted and respiratory disease, cardiovascular disease, diabetes, immunocompromised, SOFA score and clinical frailty scale were included in the final model; Hosmer–Lemeshow test yielded a nonsignificant p value of 0.458.
Table 3
| Variable | Low risk (n = 24)a) | Intermediate risk (n = 55)a) | High risk (n = 125)a) | p value |
|---|---|---|---|---|
| Progression to critical illness | 0 (0) | 1 (1.8) | 36 (28.8) | < 0.001 |
| 28-day mortality | 0 (0) | 1 (1.8) | 16 (12.8) | 0.017 |
| Discharge to homeb) | 21/24 (87.5) | 46/54 (85.2) | 69/101 (68.3) | 0.023 |
| In-hospital mortality | 0 (0) | 1 (1.8) | 24 (19.2) | < 0.001 |
| Hospital length of stay (d)c) | 7 (6–10) | 10 (7–17) | 16 (8–33) | < 0.001 |
a) Risk-stratified groups: a high-risk group with two or more risk factors, intermediate-risk group with one risk factor, and low-risk group with no risk factors. The risk factors for progression to critical illness included preexisting respiratory disease, preexisting cardiovascular disease, immunocompromised, and higher clinical frailty scale (> 6.5) and sequential organ failure assessment score (> 4.5) at baseline.
REFERENCES
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 36 View
- 26 Download
- Related articles
-
A novel strategy for predicting critical illness in hospital-acquired COVID-192024 May;39(3)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement table 1
Supplement table 1 Print
Print


