 |
 |
| Korean J Intern Med > Volume 39(1); 2024 > Article |
|
Abstract
Post-tuberculosis lung disease (PTLD) is emerging as a significant area of global interest. As the number of patients surviving tuberculosis (TB) increases, the subsequent long-term repercussions have drawn increased attention due to their profound clinical and socioeconomic impacts. A primary obstacle to its comprehensive study has been its marked heterogeneity. The disease presents a spectrum of clinical manifestations which encompass tracheobronchial stenosis, bronchiectasis, granulomas with fibrosis, cavitation with associated aspergillosis, chronic pleural diseases, and small airway diseases—all persistent consequences of PTLD. The spectrum of symptoms a patient may experience varies based on the severity of the initial infection and the efficacy of the treatment received. As a result, the long-term management of PTLD necessitates a detailed and specific approach, addressing each manifestation individually—a tailored strategy. In the immediate aftermath (0–12 months after anti-TB chemotherapy), there should be an emphasis on monitoring for relapse, tracheobronchial stenosis, and smoking cessation. Subsequent management should focus on addressing hemoptysis, managing infection including aspergillosis, and TB-associated chronic obstructive pulmonary disease or restrictive lung function. There remains a vast expanse of knowledge to be discovered in PTLD. This review emphasizes the pressing need for comprehensive, consolidated guidelines for management of patients with PTLD.
According to the World Health Organization (WHO) reports, about 74 million lives were saved through tuberculosis (TB) diagnosis and treatment between 2000 and 2021 [1]. Unlike the previous years in the early 20th century, advancements in the more effective and shorter duration anti-TB treatment, coupled with a more systemic public health system worldwide, have resulted in increased survival among TB patients [2,3]. Consequently, the terminology “post-tuberculosis lung disease (PTLD)” has emerged. In 2019, the first International Post-Tuberculosis Symposium was convened, where physicians worldwide discussed PTLD, defined as a chronic respiratory abnormality partially attributed to previous pulmonary TB [4–6]. As TB survival rates increase, the socioeconomic impact of survivors and the associated attention to PTLD intensify.
This review employs a hybrid approach, merging an umbrella review of existing systematic reviews on individuals with PTLD and a comprehensive narrative review to address underexplored areas in the current literature [7–10]. We commence by outlining the pathogenesis and epidemiology of pulmonary TB, based on recent reviews. Given our heightened interest in prospective and detailed studies elucidating the variability among survivors of pulmonary TB (or PTLD patients), we conducted an additional narrative review of references in PubMed published up to August 2023, using the search terms “pulmonary tuberculosis”, “epidemiology”, and “pathogenesis”. Subsequent umbrella reviews delving into the long-term mortality of PTLD and its clinical management, supplemented with additional narrative reviews incorporating search terms like “pulmonary tuberculosis”, “tracheobronchial stenosis”, “endobronchial tuberculosis”, “pulmonary function”, “bronchiectasis”, “pulmonary aspergillosis”, “cavitation”, “hemoptysis”, “rehabilitation”, and “vaccination” enabled us to gain a holistic understanding of PTLD. This methodology was particularly necessary given that emphasis on PTLD has only recently increased. We found that there is a limited number of systematic reviews available for quality assessment or comparison, and there is a notable shortage of randomized controlled trials that address the various subtypes of PTLD, including bronchiectasis, cavitation, aspergillosis, chronic obstructive pulmonary disease (COPD), and tracheobronchial stenosis—all persistent outcomes of PTLD [4,11–16]. This review delves into the multifaceted nature of PTLD, its personalized assessment and management strategies, and points towards future research directions.
The pathogenesis of TB commences with the inhalation of Mycobacterium tuberculosis droplets that reach the alveoli. After being phagocytosed by alveolar macrophages, the bacterium evades elimination and replicates within them [17]. The infected macrophages are soon destroyed, and nearby immune cells, in conjunction with migrated T lymphocytes activated by antigen-presenting cells within 6–8 weeks post-infection, form tuberculous granulomas [18]. The disease’s primary progression stems from this granuloma formation. The extent of lung damage, however, hinges on the host’s immune response, which can differ due to genetic variations [10]. This variation forms the foundation of PTLD’s heterogeneity.
Extensive research, including animal studies, has unveiled various factors shaping the host’s immune response to TB. A study employing immunohistochemical staining on granulomas from diverse TB patients revealed significant variations in immune reactivity. While macrophages and multinucleated giant cells were consistent in number across samples, T lymphocyte distribution varied [19]. Cytokine expression and release can differ among patients. For instance, tumor necrosis factor alpha (TNF-α) aids in countering TB by fostering granuloma formations. However, its premature or excessive secretion can induce an intensified inflammatory response, further damaging lung tissues [19]. A study comparing TB patient sera indicated elevated TNF-α levels in severe cases than in mild-to-moderate ones (p < 0.05) [20]. Matrix metalloproteinases (MMPs), encompassing 25 potent proteases, play a pivotal role in lung remodeling during TB. Elevated MMP-1 gene expression in sputum and bronchoalveolar lavage fluid samples was found to correlate with alveolar destruction in lung granulomas and increased collagen breakdown [21]. Interestingly, MMP-1 concentrations were 8.5-fold higher in patients with pronounced lung disease compared to those with milder afflictions [21]. Additionally, a study showed that the median value of serum MMP-1 was significantly higher in post-TB patients with sequelae than in post-TB patients without sequelae [22]. Owing to the distinctive characteristics of granulomas, cavitations, and fibrosis, which are further influenced by unique healing capacities and external environmental factors such as tobacco smoking, pollution, and concomitant viral/bacterial infections, the pathogenesis of TB exhibits pronounced heterogeneity. The subsequent section delves into the prevalent features of PTLD, given its diverse clinical manifestations.
Historically, TB research predominantly focused on its acute phase and therapeutic management, sidelining the prolonged post-TB phase (or PTLD) that carries considerable socioeconomic implications. However, in recent years, PTLD’s global significance has become increasingly apparent. A systematic review and meta-analysis examining long-term all-cause mortality in PTLD, which included 40,781 individuals from various countries such as Vietnam, India, China, and Ethiopia, showed that PTLD patients even with confirmed treatment completion had a 3.76 times higher rate of all-cause mortality compared to the general population [7]. The global trend of declining estimated TB deaths and mortality rates witnessed a reversal in 2020 with an estimated 1.5 million deaths, and further increased in 2021 to 1.6 million deaths, up from 1.4 million in 2019. This escalation is anticipated to amplify the burden of PTLD [1].
In a pivotal move, the recent symposium in 2019 (https://www.post-tuberculosis.com) highlighted PTLD as an emerging issue of interest and first defined it as “evidence of chronic respiratory abnormality, with or without symptoms, attributable at least in part to previous pulmonary tuberculosis” [6]. This suggests that PTLD encompasses a spectrum of post-infectious sequelae, and TB survivors potentially fall under the PTLD category [15]. According to Menzies et. al.[23], the estimated disability-adjusted life-years stood at 12.1 per TB case, with 28% spanning 15 or more years following the initial TB episode. A systemic review on long-term, all-cause mortality in PTLD indicated that the combined standardized mortality ratios for individuals with confirmed treatment completions or cures, in comparison to controls, stood at 3.76 [7]. Remarkably, even in high-income countries, fully treated TB remained associated with a heightened mortality risk. There was a marked increase in all-cause deaths among post-treatment TB patients compared to controls (20.7 vs. 3.1%) [24]. A recent cohort study involving 82,098 adult TB survivors showed a 1.62-fold higher mortality risk in TB survivors compared to controls, even after adjusting for potential confounders [25]. Given that TB pathophysiology implicates both the small and large airways, lung parenchyma, pleura, and even pulmonary vessels, the clinical manifestations of PTLD are varied. This variation can even be discerned from one lobe to another within a single patient, adding layers of complexity to the disease profile [26,27]. Consequently, understanding the epidemiology of PTLD proves challenging and remains an area awaiting further elucidation.
Airway-related morbidities can be classified into those affecting the large airways, including the trachea, main bronchi, and bronchi, and those involving the small airways, specifically those less than 2 millimeters in diameter. In the acute phase of infection, the anatomy of these airways may be stenotic, distorted, or enlarged due to extensive wall injuries. Damage to the small airways often results in airflow limitation, which can be quantified using spirometry (Table 1).
Endobonchial tuberculosis (EBTB) incidence is reported to range from 10% to 38.8% among TB patients [28]. Intriguingly, in South Korea, EBTB demonstrates a predilection for younger female TB patients, specifically those in their 20s and 30s [28–30]. To establish a diagnosis, bronchoscopic examination, complemented by microbial and histopathological confirmations, is essential. Subsequent computed tomography (CT) scans are also recommended, as EBTB often eludes detection in plain chest radiographs [31]. Hallmark bronchoscopic features encompass actively caseating, edematous-hyperemic, fibrostenotic, tumorous, and ulcerative types (Fig. 1). While most lesions demonstrated improvement within a 5-month treatment period, around two-thirds of those with actively caseating and edematous-hyperemic types metamorphosed into the fibrostenotic type after anti-TB therapy [32]. The concomitant use of corticosteroids with anti-TB treatment to avert tracheobronchial stenosis remains a subject of debate for adult EBTB patients. However, initiating corticosteroid treatment during the early phase (symptoms lasting less than 3 months) may offer therapeutic benefits [33,34].
Despite therapeutic efforts, between 60 and 95% of cases exhibited tracheobronchial stenosis or strictures to varying degrees. While there is some variability between studies, common sites of affliction include the right and left main bronchi (87%), right upper lobe bronchus (65%), and the trachea (50%) (Fig. 2). Conducting bronchoscopic assessments during and after anti-TB therapy can aid clinicians in identifying further progression of stenotic complications. When irreversible EBTB complications manifest, clinicians might consider interventional bronchoscopy and surgical interventions, as discussed in subsequent sections.
Bronchiectasis is defined as permanently dilated airways due to chronic bronchial inflammation caused by inefficient microbial clearance and recurrent or persistent infections (Table 1) [35]. While the predominant infectious causes of bronchiectasis differ globally, TB stands out as one of the principal post-infectious etiologies, especially in countries with a high prevalence of TB [36–39]. A cross-sectional study, drawing on data from the Korean National Health and Nutrition Examination Survey from 2007–2009, revealed 43.5% of bronchiectasis patients had a prior history of pulmonary TB (Fig. 3A). To put this into perspective, approximately half of bronchiectasis patients in Korea can be classified as PTLD patients with subsequent complications. Bronchiectasis, like EBTB, is also recognized as an evolving manifestation during the course of TB infection. Notably, CT scans have shown emergent bronchiectasis in roughly a quarter of active TB patients [40]. Bronchiectasis develops secondary to adjacent parenchymal destruction and fibrosis, leading to traction bronchiectasis (Fig. 3A). However, it can also develop in conjunction with EBTB due to tracheobronchial stenosis with its distal bronchial dilatation, which subsequently becomes bronchiectasis.
Hemoptysis is frequently associated with bronchiectasis, occurring in 22–25% of afflicted patients [36,37]. A markedly higher proportion of bronchiectasis patients admitted due to hemoptysis have a history of pulmonary TB, with an odds ratio (OR) of 1.66 (95% confidence interval [CI], 1.03–2.69; p = 0.038) [41]. The increased prevalence of hemoptysis in post-TB bronchiectasis isn’t yet fully understood. However, this group often exhibits marked reductions in lung function, as demonstrated by diminished forced expiratory volume in one second (FEV1) and greater evidence of bronchial artery hypertrophy—a major hemoptysis contributor [41–44]. It’s conceivable that severe symptoms, such as consistent coughing and sputum production, could intensify pressures on nearby blood vessels. This may heighten the likelihood of vessel rupture, leading to hemoptysis.
Obstruction of small airways can be quantified post-bronchodilator using spirometry. A population-based multicenter, cross-sectional study, the Burden of Obstructive Lung Disease (BOLD) with 14,050 participants, established a significant association between a history of TB and spirometry-verified airflow obstruction (adjusted OR, 2.51; 95% CI, 1.83–3.42) and also restriction (adjusted OR, 2.13; 95% CI, 1.42–3.19) [16]. There is a broad spectrum of residual spirometric abnormalities among people with PTLD, often displaying a combined obstructive and restrictive pattern [10]. Because spirometry results simply represent the combined expiratory airflows from the entire lungs, measured at the end of the blowing tip by the flow detector, COPD patients with PTLD and those with COPD due to tobacco smoking might exhibit similar spirometric outcomes despite their anatomical and pathophysiological differences (Table 2) [45]. While tobacco-induced COPD typically affects all lung lobes uniformly, TB-associated COPD may afflict a selective number of lobes based on the primary infection site and its progression during the infectious phase and treatment duration (Fig. 4).
Granulomas are the hallmark of TB, and multiple granulomas are frequently observed in TB patients, even several years following the completion of anti-TB therapy (Fig. 3B). As TB progresses, mononuclear inflammatory cells form the core structure of granulomas, undergoing cellular metamorphosis to yield epithelioid cells, multinucleated giant cells, and foamy macrophages [46]. Furthermore, granulomas incorporate other immune cells, including T and B lymphocytes and fibroblasts. While much remains to be elucidated, animal studies infected with TB suggest that each individual granulomatous lesion may uniquely respond to the host’s immune reaction, potentially leading to varied disease trajectories [47,48]. The fibrotic transformation of granulomas is believed to involve collagen-producing fibroblasts and pro-fibrotic cytokines such as transforming growth factor beta 1 (TGF-β1) [49]. This fibrotic encapsulation of granulomas can benefit the host by segregating infected tissues from healthy ones [50]. Nonetheless, excessive fibrotic progression can be detrimental, with traction bronchiectasis as a good example.
Subsequent to completing anti-TB treatment, characteristic CT imaging of early TB manifestations like marginated linear branching centrilobular nodules (referred to as the “tree-in-bud” pattern) and poorly defined heterogeneous consolidations resolve and diminish both in size and density [51]. During treatment, the “tree-in-bud” lesions disappear, leaving tiny discrete dots. Lobular consolidations resolve and turn to centrilobular nodules, which then further diminish, resulting in residual fibrotic alterations (Fig. 5). In PTLD, radiological findings often include bronchial wall thickening, emphysematous changes, fibrocalcific changes, broncho-vascular distortion, and bronchiectasis [51].
According to the widely cited experimental rabbit model, a TB cavity initially forms when the necrotic core of a granulomatous abscess erodes an adjacent airway, creating an oxygen-rich environment optimal for TB bacilli proliferation. This environment manifests as a significantly increased bacillary burden at the inner cavity edge [52–54]. Both higher rates of bacterial replication and poorly vascularized and shielded structure of the cavity can elevate the risk of treatment failure and the emergence of drug resistance [18,55–57]. Though most cavities heal and close after anti-TB chemotherapy, some persist beyond treatment. Persisting cavities post six months of treatment can double the relapse rate in comparison to healed cavities [55]. Incomplete healing might leave the cavity’s inner space exposed to the primary airway system, raising the susceptibility for secondary colonization by pathogens such as Aspergillus fumigatus.
When inhaled, Aspergillus species from the environment can lead to invasive fungal pneumonia and inflammatory necrosis, culminating in chronic pulmonary aspergillosis (CPA) [13,58]. The most prevalent type of CPA is chronic cavitary pulmonary aspergillosis (CCPA), characterized by one or more lung cavities, which may contain aspergillomas, commonly referred to as a “fungal ball” (Fig. 6) [59]. If left untreated, CPA can lead to increased vascularization of adjacent bronchial arteries, resulting in potentially fatal hemoptysis. Furthermore, untreated CPA can evolve into chronic fibrotic pulmonary aspergillosis (CFPA), a severe form marked by extensive fibrosis and the destruction of at least two lung lobes, causing significant lung function impairment [59]. CPA patients who have diabetes mellitus, malnutrition, alcoholism, are of advanced age, have undergone prolonged corticosteroid treatment, have certain immune deficiencies, connective tissue disorders, or have received radiation therapy require close medical monitoring. This is because their condition can worsen to subacute invasive aspergillosis (SAIA), a fast-progressing type of CPA where hyphae are seen invading lung tissues [13,60,61]. Conversely, in hyper-reactive (or allergic) individuals, pulmonary aspergillosis might result in allergic bronchopulmonary aspergillosis or asthma coupled with fungal sensitization [13].
Understanding post-TB patients is crucial, as these individuals may encounter complications not just in the lungs, but also cardiovascular/pericardial, neurological, psychological, and even developmental issues when the TB patient is an adolescent or child. A recent review article by Nightingale et al. highlighted the significance of post-TB management and provided comprehensive suggestions for overseeing the health and wellbeing of these patients [8]. Our review aims to focus on the specific management of post-TB patients dealing with impaired lung anatomy and physiology (or PTLD), following the successful completion of their anti-TB regimen. A predominant challenge in providing appropriate long-term care for PTLD patients is the heterogeneity of the disease. As demonstrated in Table 1, we advocate for a management strategy that categorizes PTLD into subtypes such as: 1) mild abnormal X-rays features like granulomas and fibrosis, 2) bronchiectasis, 3) cavities, 4) tracheobronchial stenosis, 5) pleural complications, and 6) small airway diseases. It is common for patients to exhibit multiple subtypes. Our proposed flowchart (Fig. 7) aims to guide clinicians through each subtype, ensuring comprehensive and thorough long-term management of PTLD. We delve into these in the subsequent sections.
TB recurrence is identifiable when a patient, after receiving at least 6 months of anti-TB drugs and confirmed smear or sputum culture negative after the treatment, manifests active TB again. The two primary causes for TB recurrence are relapse and reinfection. A true relapse occurs when TB bacilli, having survived a course of anti-TB treatment confirmed as successful, re-emerge. Thus, relapse and treatment failure can be considered distinct manifestations of a similar underlying issue [9]. Conversely, reinfection refers to a previously cured patient becoming infected with a new strain of M. tuberculosis, leading to the development of pulmonary TB again. Differentiating between reinfection and relapse necessitates genotyping, which, unfortunately, is not universally accessible across countries [62]. Recognized risk factors for relapse encompass: 1) pre-existing drug resistance, 2) adoption of alternative therapeutic regimens excluding rifampicin, 3) extensive residual radiological abnormalities, such as cavitations or the presence of scars in three or more zones, 4) advanced age, 5) being male, and 6) habits like smoking or alcohol consumption [63–68]. Studies indicate that relapses predominantly arise within 6–12 months post-treatment completion [69]. Therefore, it is imperative for people with PTLD to undergo more frequent follow-ups during the initial year. Subsequent clinical visits should be strategically scheduled based on the specific sequelae warranting attention in the patient.
TB, a leading cause of benign tracheobronchial stenosis, often results in life-threatening and life-long complications in PTLD patients. Mild luminal narrowing may not mandate rigorous intervention beyond the administration of mucolytics. In contrast, complete obliteration, which leads to a significant reduction in airflow and subsequent respiratory failure, has the potential to be lethal [12]. The left main bronchus and left bronchi are typically more prone to involvement, largely attributed to the added anatomical compression exerted by the aortic arch (Fig. 2) [70–72]. Historically, prior to the 1990s, surgical resection and bronchoplastic reconstruction were the standard treatments for tracheobronchial stenosis [71]. The advent of the late 1990s saw the introduction of less invasive techniques utilizing rigid bronchoscopy. These can be broadly categorized into dilatation and ablation. These techniques emerged as superior alternatives for patients with multiple stenosis sites, especially when accompanied by severe comorbidities such as heart failure or chronic renal disease. Although no studies have directly contrasted the efficacy of these diverse bronchoscopic procedures, the balloon dilation technique has gained preference. Its minimally invasive nature, coupled with the need for fewer specialized skills and the widespread availability of bronchoscopes, makes it the often-chosen treatment. The procedure entails positioning a deflated balloon within the stenotic bronchus, which is then inflated radially for several seconds to minutes (Fig. 8) [73].
Recently, the dilation process has incorporated multiple modalities, including laser resection and mechanical bougienation, typically performed under general anesthesia [74]. Because balloon dilation often prove ineffective, stent insertion is typically the subsequent preferred intervention. Airway stenting is valuable not only post balloon dilation failure but also in scenarios complicated by tracheobronchomalacia or fistulas, conditions not suitable for balloon dilation [12]. Given the complications associated with metallic stents (e.g., metal fracture, granulation tissue proliferation), there is a shift towards favoring silicone stents [71,75–77]. Research centered on silicone stents highlights that a majority of patients (ranging from 63.6 to 100%) experience immediate symptom alleviation post-placement [72,78]. A recent study analyzed 381 post-TB tracheobronchial stenosis patients who underwent silicone stenting. The results revealed that 75% (or 285 patients) successfully had their stents removed after a median duration of 25 months, achieving a 70% stent-free rate [74]. However, prolonged placement of silicone stents can also lead to granulation formation [72,79,80].
Hemoptysis can often prove fatal. PTLD patients, especially those with bronchiectasis or cavitation, are particularly susceptible to hemoptysis [81]. Although previously discussed, the precise mechanism underlying the increased incidence of hemoptysis in post-TB bronchiectasis remains elusive. Furthermore, the current therapeutic approach to hemoptysis in bronchiectasis does not specifically address its post-TB variant. It’s noteworthy that while 99% of lung blood flow originates from pulmonary arteries, primarily facilitating gas exchange, an overwhelming 90% of life-threatening hemoptysis instances are attributable to the bronchial arteries [82]. In PTLD patients, factors such as bronchiectasis, pulmonary hypertension, chronic infection, and an aberrant vasculature elevate the risk of bronchial artery rupture. These conditions may precipitate a redirection of blood flow from the pulmonary to bronchial arteries, ensuing in bronchial artery dilation, hypervascularization, and the genesis of collateral vessels that are rupture-prone [83].
For instances where hemoptysis is quiescent and minimal, administration of tranexamic acid, an antifibrinolytic agents, is often deemed appropriate. This can be delivered via nebulization, intravenous infusion, or oral intake. While robust evidence remains pending, certain studies suggest potential benefits, such as reductions in hemoptysis volume, shorter hospitalization durations, and diminished needs for invasive interventions, all with minimal side effects compared to controls [84–86]. Moreover, should the hemoptysis source be pinpointed, positioning the patient in a decubitus stance with the afflicted side downward can deter the aspiration of blood into the unaffected lung [14]. In the event of significant or life-endangering hemoptysis, immediate referral to an emergency setting is imperative. Intensive care interventions could encompass endotracheal intubation, bronchoscopic management, and the more decisive measure of angiography followed by bronchial artery embolization. Upon angiographic identification of the bleeding vessel, arterial embolization is typically executed utilizing microparticle embolic agents such as polyvinyl alcohol particles or tris-acryl gelatin microspheres [87,88].
Infective exacerbation is another complication frequently observed in PTLD patients with bronchiectasis. A robust correlation exists between chronic bacterial infection and exacerbation of bronchiectasis [89–91]. Notably, Pseudomonas aeruginosa has been identified in numerous bronchiectasis patients, showing a prevalence rate of 15.0%. This bacterium has also been independently linked with heightened mortality (hazard ratio [HR], 2.03; 95% CI, 1.36–3.03; p = 0.001) among those experiencing regular exacerbations [92]. A reduction in microbiome diversity that leads to Pseudomonas dominance is also associated with greater disease severity and higher risk of mortality [93]. Consequently, antibiotics emerge as a pivotal therapeutic intervention for infective exacerbations of bronchiectasis. PTLD patients with bronchiectasis should thus receive similar treatment. Typically, a 14-day antibiotic regimen is administered to counter exacerbations involving P. aeruginosa [11,94,95]. Recently, guidelines and reports have highlighted the importance of comprehensive management of bronchiectasis to prevent exacerbations using non-pharmacological treatments, such as airway clearance techniques and pulmonary rehabilitation (PR) [96]. While comprehensive research is necessary to fully understand the specifics of PTLD exacerbation, we suggest that PTLD patients with bronchiectasis be managed analogously when experiencing infective exacerbations.
Surgical resection of cavitary TB, a potential precursor to drug-resistant TB, remains an option but typically presents a poor prognosis. The majority of surgical treatments were explored in the early 20th century, prior to the advent of effective chemotherapy [97]. The WHO consolidated guidelines advocate for elective partial lung resection in patients with rifampicin-/multidrug-resistant-TB [98].
A cavity with fungal ball, particularly due to Aspergillus, can lead to symptoms such as hemoptysis, chest pain, cough, and fever. The management strategies for CPA differ based on the disease’s severity and type, encompassing simple aspergilloma, CPPA, CFPA, and SAIA. Before initiating treatment for CPA, obtaining a definitive diagnosis is crucial. This diagnosis is based on several key criteria. First, thoracic imaging must show the presence of one or more cavities, which can either contain a fungal ball or not, or demonstrate the presence of nodules. Second, there should be direct evidence of an Aspergillus infection, as determined by either microscopy or a culture derived from a biopsy. Alternatively, there can be a clear immunological response to Aspergillus species. Third, other potential diagnoses must be excluded. Lastly, these symptoms or findings should have been consistent for at least three months [59]. For example, a PTLD patient’s CT scan showing a fungal ball present for over three months, coupled with a positive Aspergillus IgG or precipitins test (noted in over 90% of cases), suffices for CPA and, more specifically, CCPA confirmation. CFPA refers to CPA with extensive fibrosis affecting at least two lobes, while SAIA denotes rapidly-progressing CPA, usually within three months, with histologic evidence of invasive hyphae in lung tissue, especially among the immunocompromised or severely debilitated as previously described.
Simple aspergilloma, rounded accumulations of fungal hyphae, fibrin, mucus, and cellular debris, poses a 15–20% risk of development in cavities larger than 2 cm [13]. For PTLD patients with cavities exceeding 2 cm, regular monitoring for the emergence of aspergilloma is advised. Upon confirmation of a simple aspergilloma, surgical resection becomes the often-recommended intervention to counter and treat potentially lethal hemoptysis [59]. On the other hand, CCPA generally demands oral triazoles therapy to prevent both its clinical and radiological symptoms, notably fibrosis and severe hemoptysis [99–105]. For non-severe symptoms, outpatient oral azole therapy (e.g., itraconazole 200 mg or voriconazole 150–200 mg twice daily for a minimum of 4–6 months) can be considered. In cases where SAIA is suspected, PTLD patients should be immediately referred to a tertiary care hospital for prompt diagnosis, utilizing tools like CT scans and bronchoscopy. The condition should be treated similarly to acute invasive aspergillosis because of its rapid progression and high mortality rate.
Although smoking is the traditional risk factor for COPD, TB or PTLD has been identified as another significant and independent risk factor for airflow obstruction. Conversely, smoking is also a risk factor for TB development and can exacerbate its severity [106]. A comprehensive cross-sectional survey from China in 2021 demonstrated that of 610 confirmed PTLD patients, 21.3% experienced airflow obstruction. Even after accounting for confounding factors, a history of TB remained a significant correlate of airflow obstruction (OR, 1.31), and compromised lung function persisted [107]. A prospective study from Malawi on PTLD encompassed 301 patients (60.4% HIV positive) who, post-active TB treatment and a subsequent 3-year follow-up, 27.9% maintained compromised lung function, with a median FEV1 decline of 180 mL (or 60 mL/yr) [108]. Interestingly, the post-hoc analysis revealed a marked improvement in lung function during the first 9 months following treatment completion, with fewer changes observed thereafter. Hence, the importance of baseline lung function assessment and subsequent annual follow-ups is emphasized to identify deteriorating subgroups for timely intervention.
What predisposes individuals to compromised lung function and airflow obstruction? A landmark study conducted in Hong Kong, incorporating 16,345 active TB patients, revealed that both current and former smokers were associated with more pronounced lung disease, cavitation, and a 1.5–2 times increased likelihood of remaining smear- and culture-positive following two months of treatment. These individuals were also less probable to attain a cure or complete treatment within two years and had elevated relapse rates compared to non-smokers [109]. This underscores the pressing need for physicians to advocate for smoking cessation among PTLD patients.
A pivotal question emerges: Are the COPD cases induced by smoking and TB, both resulting in chronic airflow obstruction, distinct pathological entities? As previously described, these COPD subtypes manifest unique physiological and radiological attributes (Table 2). In recent years, there’s a shifting perspective to view COPD as a continuum of airflow obstruction with various endotypes, of which TB is one [110]. This aligns with the contemporary stances adopted by both the Global Initiative for Chronic Obstructive Lung Disease and Lancet committees [111]. While further research is essential to discern the optimal treatment modalities for PTLD versus conventional smoking-induced COPD, inhaler therapies appear comparably efficacious in PTLD. Despite the absence of long-term randomized trials confirming efficacy, both long-acting bronchodilator therapies (like long-acting β-agonist [LABA] and long-acting muscarinic antagonist [LAMA]) have shown promise in alleviating TB-associated COPD symptoms and improving lung function. A trial of LABA (inhaled indacaterol 150 μg once daily) targeting PTLD with moderate-to-severe airflow restriction revealed enhanced symptom scores and a significant increase in trough FEV1 by 140 mL (p < 0.001) in comparison to a placebo at the 8-week mark [112]. Though the sample was limited (n = 29), a study on the impact of LAMA on PTLD indicated that 8-week daily inhaled tiotropium bromide administration improved their lung function [113]. However, the pulmonary function patterns in many TB-associated COPD cases are varied, and which specific phenotypes respond best to long-acting bronchodilator therapies remains undetermined.
Conversely, the use of inhaled corticosteroids (ICS) is discouraged in TB-associated COPD. While ICS/LABA therapies have demonstrated efficacy in certain COPD populations, numerous studies have highlighted the potential long-term adverse effects of ICS, specifically increased risks of TB and mycobacterial diseases in this demographic [114]. ICS use in COPD patients may increase the risk of pneumonia by suppressing both cellular and humoral immunity, altering the bactericidal effect of macrophages, and reducing nitric oxide production [115]. Such risks seem contingent on the specific ICS type. Research from Taiwan involving TB-associated COPD patients revealed that those in the fluticasone/salmeterol cohort exhibited nearly a 50% increased likelihood of developing active TB relative to the budesonide/formoterol group (adjusted HR, 1.45; 95% CI, 1.14–1.85) [116]. Again, further investigations are warranted to elucidate the fundamental mechanisms relating ICS use to TB relapse.
PR has established itself as a cornerstone in the management of chronic respiratory diseases (CRD) including asthma, COPD, cystic fibrosis, and others [117,118]. It should be a personalized, multidisciplinary program tailored to individual patient needs [117]. Furthermore, PR has been demonstrated to be more cost-effective than pharmacotherapy in patients with CRD [119]. However, it’s pivotal to emphasize that for a program to be classified as PR, it must encompass comprehensive baseline and subsequent post-PR outcome measurements to validate its efficacy. While there is a robust body of evidence supporting PR in general CRD, specific research addressing PTLD remains more limited [120]. Nonetheless, there is emerging evidence regarding PR programs uniquely tailored for PTLD patients [121–124].
We list recommendations for PTLD-specific PR as follows [125–130]. Firstly, aerobic exercise is advised, using either a treadmill or cycle-ergometer for 30 minutes, 2–5 times a week over a span of 4–8 weeks. Depending on the patient’s initial physical assessment, this may be replaced with free walking. Secondly, airway clearance techniques such as gravity-assisted drainage, various breathing strategies, directed coughing, and positive expiratory pressure are essential. Thirdly, patients should receive both nutritional guidance and psychological support. Lastly, weight-lifting and inspiratory muscle training programs are suggested, although evidence regarding their efficacy in PTLD remains limited.
While differing in their specific emphases, all articles addressing PTLD advocate for a standardized baseline study following the successful treatment of active TB. They also emphasize active follow-up and timely interventions for PTLD patients when deemed necessary. Drawing on the most recent research and review articles, we present a clear and conventional flowchart to guide clinicians across various specialties in the long-term management of PTLD patients upon their initial presentation to clinics or hospitals (Fig. 7). It is pertinent to note that the flowchart is based on limited existing evidence, underscoring the need for further research to refine our proposed standards.
We also advocate for future research on PTLD to focus on the following areas. First, we need to establish the risk factors for severe PTLD and the associated poor health outcomes, such as mortality. Next, it is necessary to develop optimal approaches and algorithms for diagnosing and managing PTLD. For instance, research aimed at elucidating the mechanisms that contribute to the increased incidence of hemoptysis among post-TB bronchiectasis patients is crucial, as this will guide more effective preventive strategies and management plans. It’s vital to determine which specific phenotypes of TB-associated COPD respond best to long-acting bronchodilator therapies. Additionally, a thorough risk-benefit analysis of ICS therapies is essential to refine treatment approaches for PTLD. Lastly, we must identify cost-effective methods to deliver PR and explore the impact of patient education programs on long-term health outcomes.
Acknowledgments
We extend our sincere thanks to Dr. Hyeong Jun Cho, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, for generously providing the photograph shown in Figure 8.
Notes
CRedit authorship contributions
Wan Seo: conceptualization, methodology, investigation, writing - original draft, visualization; Hyung Woo Kim: conceptualization, methodology, supervision; Ju Sang Kim: conceptualization, methodology, supervision; Jinsoo Min: conceptualization, methodology, validation, writing - review & editing, supervision, funding acquisition
Figure 1
Hallmark bronchoscopic features of endobronchial tuberculosis patients. (A) Actively caseating type with a whitish cheese-like material (arrow heads), edematous-hyperemic type with swollen and hyperemic mucosal features (solid arrow), (B) fibrostenotic type with narrowing of the bronchial lumen due to fibrosis, and (C) ulcerative type with mucus secretion collected.

Figure 2
The distribution of endobronchial tuberculosis. Right main, right main bronchus; Left main, left main bronchus; RUL, right upper lobe; RI, right intermedius; RML, right middle lobe; Upper, upper division of left upper lobe; Lingular, lingular division of left upper lobe; LUL, left upper lobe. Adapted from Shim [30].

Figure 3
Computed tomography scans of post-tuberculosis lung disease patients with various clinical and radiologic features. (A) Post-tuberculosis lung disease patient with bronchiectasis (arrows) and fibrotic changes, (B) post-tuberculosis lung disease patients with multiple granulomas, remaining small nodularity, and fibrotic changes with traction of pleural, and (C) totally destroyed left upper lung with surrounding pleural wall thickenings (arrows).
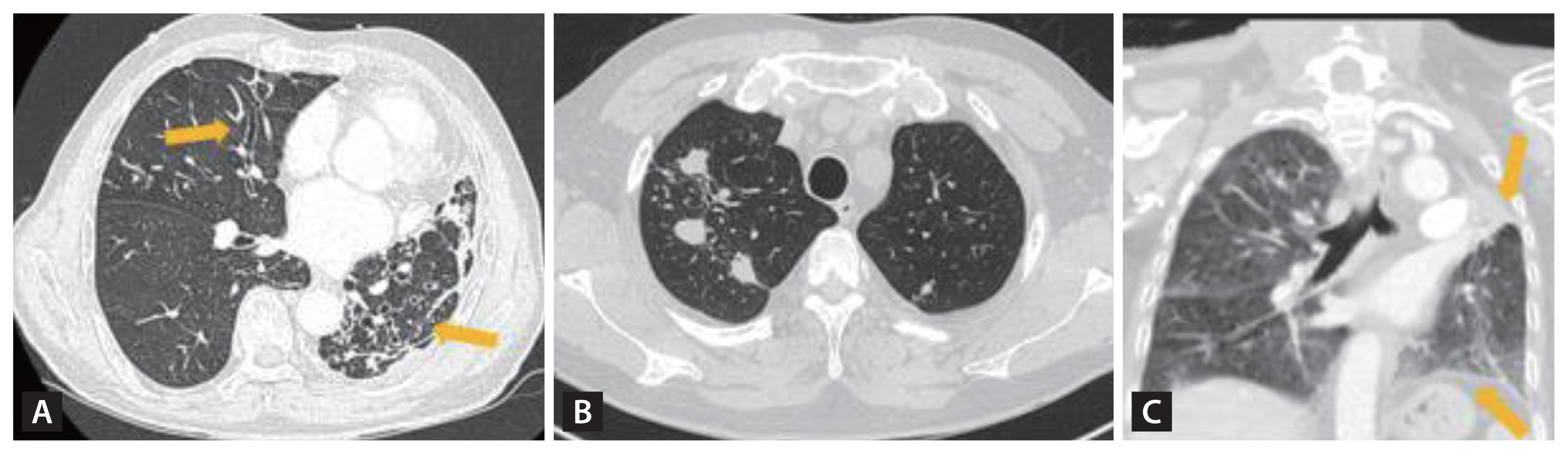
Figure 4
(A) A COPD patient with no history of TB and a 40 pack-year smoking history. CT imaging reveals signs of emphysema, bronchial wall thickening, and post-bronchodilator spirometry demonstrates an FEV1/FVC ratio of 0.46, FEV1 at 58% (1.73 L) of the predicted value, TLC at 5.46 L (84%), and DLco at 13.2 (65%) mL/min/mmHg. (B) A PTLD patient with no smoking history. CT imaging displays TB sequelae predominantly in the right upper lobe with minimal involvement in the other lobes. Pulmonary function tests do not indicate any decline, with a post-bronchodilator spirometry showing an FEV1/FVC ratio of 0.76, and FEV1 at 93% (3.02 L). (C) A PTLD patient with no smoking history. CT imaging shows a completely destroyed left lung, calcified granulomas, and fibrotic scars in the right upper lobe with pulmonary function significantly diminished, with an FEV1/FVC ratio of 0.55, FEV1 at 25% (0.59 L), TLC at 60% (2.81 L), and DLco at 12.0 (68%) mL/min/mmHg. COPD, chronic obstructive pulmonary disease; TB, tuberculosis; CT, computed tomography; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; TLC, total lung capacity, DLco, diffusing capacity of the lungs for carbon monoxide; PTLD, pulmonary-TB lung disease.

Figure 5
Pair of the same section of computed tomography scans of a pulmonary tuberculosis patient at the commencement of antituberculosis treatment (A) and after 3 months of the treatment (B).
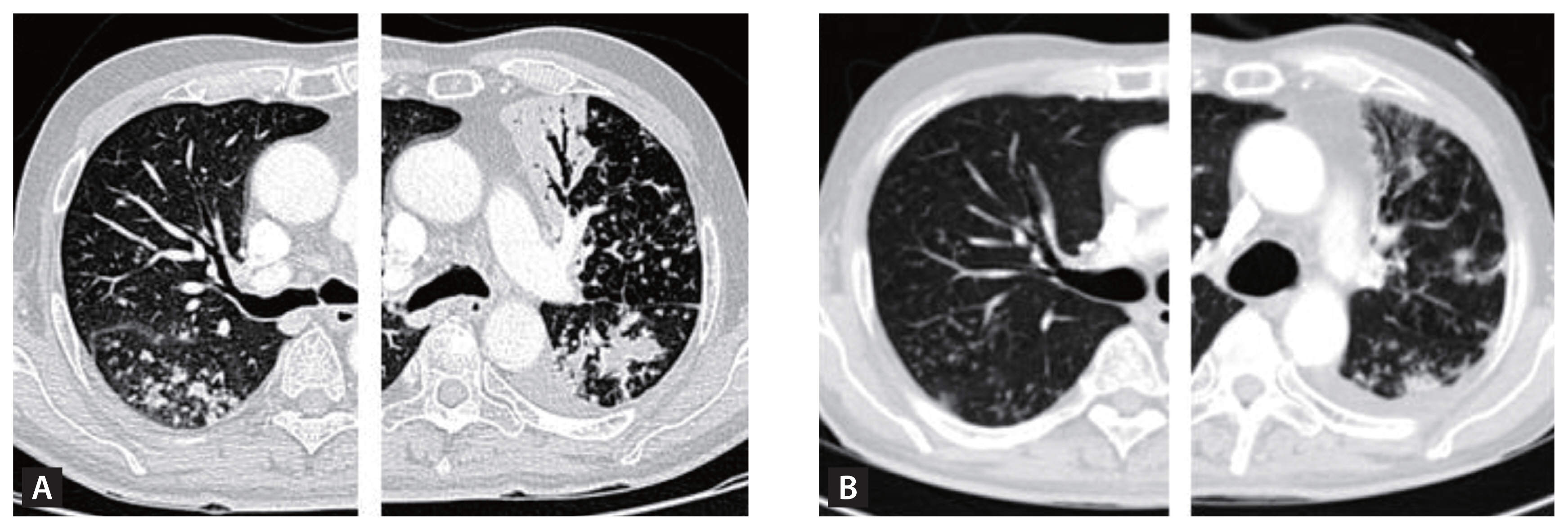
Figure 6
A PTLD patient with cavitation in the LUL presented with dyspnea, sputum, and cough. The recent CT scan revealed a newly observed mass-like lesion with an air crescent sign (A) in the previously empty cavitation seen on a CT scan 17 years ago (B), confirming newly developed Aspergillosis with suspected invasive aspergillosis. PTLD, pulmonary-TB lung disease; LUL, left upper lung; CT, computed tomography.
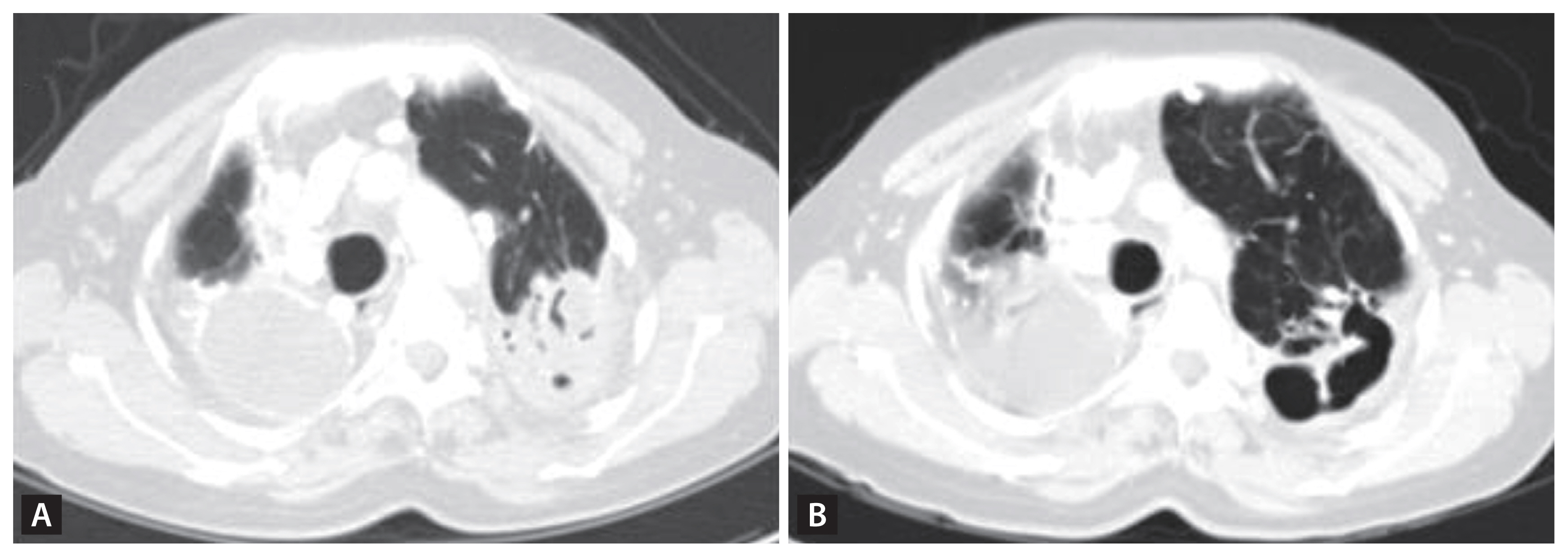
Figure 7
Flow chart of long-term management of people with post-tuberculosis lung disease. There is limited evidence to choose vaccination recommendation differently to individuals with PTLD from rest of CRD patients. TB, tuberculosis; CT, computed tomography; mMRC, modified medical research council dyspnea scale; CAT, chronic obstructive pulmonary disease assessment test; 6MWT, 6-minute walk test; SpO2, peripheral oxygen saturation; BMI, body mass index; TB-PCR, tuberculosis-polymerase chain reaction; AFB, acid-fast bacilli; COVID-19, coronavirus disease 2019; NTM, non-tuberculous mycobacterium; COPD, chronic obstructive pulmonary disease; LAMA, long-acting muscarinic antagonist; LABA, long-acting beta agonist; ICS, inhaled corticosteroids; IV, intravenous; CCPA, chronic cavitary pulmonary aspergillosis; CFPA, chronic fibrosing pulmonary aspergillosis; SAIA, subacute invasive aspergillosis.

Figure 8
Balloon dilation procedure on the annular cicatrical stenosis at the left main bronchus of an endobronchial tuberculosis patient after two years of antituberculosis chemotherapy (A) and chest X-rays before (B) and after the dilation procedure (C).
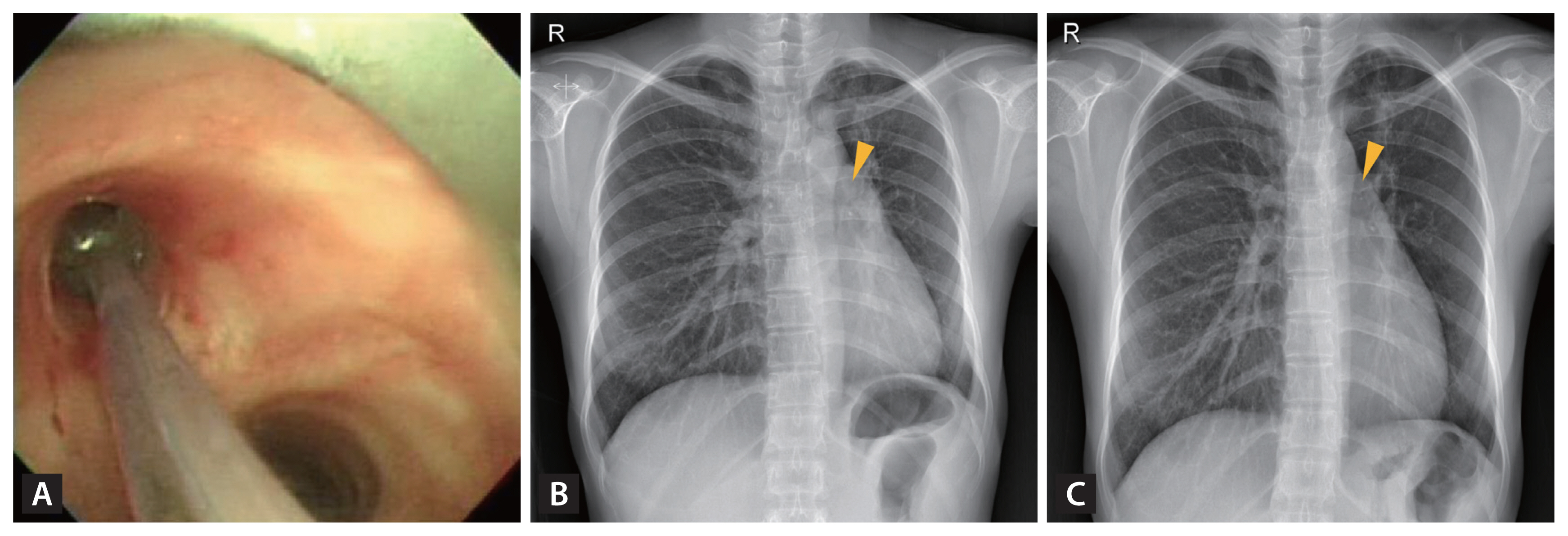
Table 1
Clinical features of post-tuberculosis lung disease based on anatomical classifications
| Anatomy | Anatomical change | Suggested definitiona) | Associated morbidity | Diagnosis/assessment |
|---|---|---|---|---|
| Large airways | Tracheobronchial stenosis | Airway obstruction primarily related to EBTB | Respiratory failure, difficult mucus clearing | CXR, CT scan, bronchoscopy |
| Bronchiectasis | Evidence of airway dilatation larger than the diameter of adjacent vessel, or non-tapering | Hemoptysis, infective exacerbation | CXR, CT scan | |
| Small airways | Increased wall thickening and narrowing of lumen | Airway obstruction (FEV1/FVC ratio) primarily related to small airway disease | Difficult gas exchange due to air flow limitation | Spirometry |
| Parenchyma | Granulomas and fibrosis | Organized aggregates of immune cells/areas of parenchymal scarring, with associated volume loss | Decreased DLco | CXR, CT scan |
| Cavitation | A gas-filled space either within an area of pulmonary consolidation or surrounded by a thin wall | CPA, IA | CXR, CT scan | |
| Pleura | Pleural thickenings | Evidence of pleural thickening on CXR or CT scan | Restrictive lung function | CXR, CT scan, spirometry, thoracoscopy |
CT, computed tomography; CXR, chest-X rays; CPA, chronic pulmonary aspergillosis; DLco, diffusing capacity of the lungs for carbon monoxide; EBTB, endobronchial tuberculosis; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; IA, invasive aspergillosis.
a) Partly adapted from Allwood et al. [15].
Table 2
The similarities and differences between PTLD and S-COPDa)
COPD, chronic obstructive pulmonary disease; PTLD, post-tuberculosis lung disease; S-COPD, smoking-associated COPD.
a) Adapted from Gai et al. [45].
REFERENCES
1. Wordl Health Organization. Global tuberculosis report 2022. Geneva: World Health Organization, 2022.
3. Snider GL. Tuberculosis then and now: a personal perspective on the last 50 years. Ann Intern Med 1997;126:237–243.


4. van Kampen SC, Wanner A, Edwards M, et al. International research and guidelines on post-tuberculosis chronic lung disorders: a systematic scoping review. BMJ Glob Health 2018;3:e000745.



5. Allwood B, van der Zalm M, Makanda G, Mortimer K, Steering Committee of the First International Post-Tuberculosis Symposium. The long shadow post-tuberculosis. Lancet Infect Dis 2019;19:1170–1171.


6. Allwood BW, van der Zalm MM, Amaral AFS, et al. Post-tuberculosis lung health: perspectives from the First International Symposium. Int J Tuberc Lung Dis 2020;24:820–828.


7. Romanowski K, Baumann B, Basham CA, Ahmad Khan F, Fox GJ, Johnston JC. Long-term all-cause mortality in people treated for tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2019;19:1129–1137.


8. Nightingale R, Carlin F, Meghji J, et al. Post-TB health and wellbeing. Int J Tuberc Lung Dis 2023;27:248–283.


9. Lambert ML, Hasker E, Van Deun A, Roberfroid D, Boelaert M, Van der Stuyft P. Recurrence in tuberculosis: relapse or reinfection? Lancet Infect Dis 2003;3:282–287.


10. Ravimohan S, Kornfeld H, Weissman D, Bisson GP. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev 2018;27:170077.



11. Choi H, Chalmers JD. Bronchiectasis exacerbation: a narrative review of causes, risk factors, management and prevention. Ann Transl Med 2023;11:25.

12. Mondoni M, Repossi A, Carlucci P, Centanni S, Sotgiu G. Bronchoscopic techniques in the management of patients with tuberculosis. Int J Infect Dis 2017;64:27–37.


13. Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax 2015;70:270–277.


14. Kathuria H, Hollingsworth HM, Vilvendhan R, Reardon C. Management of life-threatening hemoptysis. J Intensive Care 2020;8:23.




15. Allwood BW, Byrne A, Meghji J, Rachow A, van der Zalm MM, Schoch OD. Post-tuberculosis lung disease: clinical review of an under-recognised global challenge. Respiration 2021;100:751–763.



16. Amaral AF, Coton S, Kato B, et al. Tuberculosis associates with both airflow obstruction and low lung function: BOLD results. Eur Respir J 2015;46:1104–1112.



17. de Martino M, Lodi L, Galli L, Chiappini E. Immune response to mycobacterium tuberculosis: a narrative review. Front Pediatr 2019;7:350.


18. Ulrichs T, Kaufmann SH. New insights into the function of granulomas in human tuberculosis. J Pathol 2006;208:261–269.


19. Moreira AL, Tsenova L, Aman MH, et al. Mycobacterial antigens exacerbate disease manifestations in Mycobacterium tuberculosis-infected mice. Infect Immun 2002;70:2100–2107.




20. Fiorenza G, Rateni L, Farroni MA, Bogué C, Dlugovitzky DG. TNF-alpha, TGF-beta and NO relationship in sera from tuberculosis (TB) patients of different severity. Immunol Lett 2005;98:45–48.

21. Elkington P, Shiomi T, Breen R, et al. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J Clin Invest 2011;121:1827–1833.



22. Shanmugasundaram K, Talwar A, Madan K, Bade G. Pulmonary functions and inflammatory biomarkers in post-pulmonary tuberculosis sequelae. Tuberc Respir Dis (Seoul) 2022;85:175–184.




23. Menzies NA, Quaife M, Allwood BW, et al. Lifetime burden of disease due to incident tuberculosis: a global reappraisal including post-tuberculosis sequelae. Lancet Glob Health 2021;9:e1679–e1687.



24. Miller TL, Wilson FA, Pang JW, et al. Mortality hazard and survival after tuberculosis treatment. Am J Public Health 2015;105:930–937.



25. Choi H, Han K, Jung JH, et al. Long-term mortality of tuberculosis survivors in Korea: a population-based longitudinal study. Clin Infect Dis 2023;76:e973–e981.




26. Long R, Maycher B, Dhar A, Manfreda J, Hershfield E, Anthonisen N. Pulmonary tuberculosis treated with directly observed therapy: serial changes in lung structure and function. Chest 1998;113:933–943.

27. Hunter RL. Pathology of post primary tuberculosis of the lung: an illustrated critical review. Tuberculosis (Edinb) 2011;91:497–509.



28. Lee JH, Park SS, Lee DH, Shin DH, Yang SC, Yoo BM. Endobronchial tuberculosis. Clinical and bronchoscopic features in 121 cases. Chest 1992;102:990–994.


31. Hoheisel G, Chan BK, Chan CH, Chan KS, Teschler H, Costabel U. Endobronchial tuberculosis: diagnostic features and therapeutic outcome. Respir Med 1994;88:593–597.


32. Chung HS, Lee JH. Bronchoscopic assessment of the evolution of endobronchial tuberculosis. Chest 2000;117:385–392.


33. Han SW, Lee DH, Park SS, Lee JH. Clinical study of endobronchial tuberculosis. Tuberc Respir Dis 1984;31:57–61.


34. Park IW, Choi BW, Hue SH. Prospective study of corticosteroid as an adjunct in the treatment of endobronchial tuberculosis in adults. Respirology 1997;2:275–281.


36. Buscot M, Pottier H, Marquette CH, Leroy S. Phenotyping adults with non-cystic fibrosis bronchiectasis: a 10-year cohort study in a French regional university hospital center. Respiration 2016;92:1–8.



37. Aksamit TR, O’Donnell AE, Barker A, et al.; Bronchiectasis Research Registry Consortium. Adult patients with bronchiectasis: a first look at the US Bronchiectasis Research Registry. Chest 2017;151:982–992.



38. Yang B, Jang HJ, Chung SJ, et al. Factors associated with bronchiectasis in Korea: a national database study. Ann Transl Med 2020;8:1350.



39. Lee H, Choi H, Chalmers JD, et al. Characteristics of bronchiectasis in Korea: First data from the Korean Multicentre Bronchiectasis Audit and Research Collaboration registry and comparison with other international registries. Respirology 2021;26:619–621.



40. Ko JM, Kim KJ, Park SH, Park HJ. Bronchiectasis in active tuberculosis. Acta Radiol 2013;54:412–417.



41. Seo H, Cha SI, Park J, et al. Hemoptysis as the presenting manifestation of bronchiectasis-associated hospitalization in Korea. J Thorac Dis 2023;15:3636–3645.



42. Fong I, Low TB, Yii A. Characterisation of the post-tuberculous phenotype of bronchiectasis: a real-world observational study. Chron Respir Dis 2022;19:14799731221098714.




43. Kosar M, Kurt A, Keskin S, Keskin Z, Arslan H. Evaluation of effects of bronchiectasis on bronchial artery diameter with multidetector computed tomography. Acta Radiol 2014;55:171–178.



44. Marquis KM, Raptis CA, Rajput MZ, et al. CT for evaluation of hemoptysis. Radiographics 2021;41:742–761.


45. Gai X, Allwood B, Sun Y. Post-tuberculosis lung disease and chronic obstructive pulmonary disease. Chin Med J (Engl) 2023;136:1923–1928.



46. Guler R, Ozturk M, Sabeel S, et al. Targeting molecular inflammatory pathways in granuloma as host-directed therapies for tuberculosis. Front Immunol 2021;12:733853.



47. Lin PL, Rodgers M, Smith L, et al. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun 2009;77:4631–4642.




48. Lin PL, Ford CB, Coleman MT, et al. Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nat Med 2014;20:75–79.




49. DiFazio RM, Mattila JT, Klein EC, et al. Active transforming growth factor-β is associated with phenotypic changes in granulomas after drug treatment in pulmonary tuberculosis. Fibrogenesis Tissue Repair 2016;9:6.




50. Warsinske HC, DiFazio RM, Linderman JJ, Flynn JL, Kirschner DE. Identifying mechanisms driving formation of granuloma-associated fibrosis during Mycobacterium tuberculosis infection. J Theor Biol 2017;429:1–17.



51. Im JG, Itoh H, Shim YS, et al. Pulmonary tuberculosis: CT findings--early active disease and sequential change with antituberculous therapy. Radiology 1993;186:653–660.


52. Dannenberg AM. Pathogenesis of human pulmonary tuberculosis: insights from the rabbit model. Washington: ASM press, 2006.
53. Urbanowski ME, Ordonez AA, Ruiz-Bedoya CA, Jain SK, Bishai WR. Cavitary tuberculosis: the gateway of disease transmission. Lancet Infect Dis 2020;20:e117–e128.



54. Canetti G. Present aspects of bacterial resistance in tuberculosis. Am Rev Respir Dis 1965;92:687–703.

55. Benator D, Bhattacharya M, Bozeman L, et al.; Tuberculosis Trials Consortium. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet 2002;360:528–534.


56. Dartois V. The path of anti-tuberculosis drugs: from blood to lesions to mycobacterial cells. Nat Rev Microbiol 2014;12:159–167.




57. Moreno-Gamez S, Hill AL, Rosenbloom DI, Petrov DA, Nowak MA, Pennings PS. Imperfect drug penetration leads to spatial monotherapy and rapid evolution of multidrug resistance. Proc Natl Acad Sci U S A 2015;112:E2874–E2883.



59. Denning DW, Cadranel J, Beigelman-Aubry C, et al.; European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J 2016;47:45–68.

60. Denning DW, Follansbee SE, Scolaro M, Norris S, Edelstein H, Stevens DA. Pulmonary aspergillosis in the acquired immunodeficiency syndrome. N Engl J Med 1991;324:654–662.


61. Kim SY, Lee KS, Han J, et al. Semiinvasive pulmonary aspergillosis: CT and pathologic findings in six patients. AJR Am J Roentgenol 2000;174:795–798.

62. Yoo SS, Kwon JS, Kang YR, et al. The clinical characteristics and outcomes of short-term treatment in patients with recurrent pulmonary tuberculosis. Tuberc Respir Dis 2008;64:341–346.

63. Chan-Yeung M, Noertjojo K, Chan SL, Tam CM. Sex differences in tuberculosis in Hong Kong. Int J Tuberc Lung Dis 2002;6:11–18.

64. Johnson JL, Okwera A, Nsubuga P, et al. Efficacy of an unsupervised 8-month rifampicin-containing regimen for the treatment of pulmonary tuberculosis in HIV-infected adults. Uganda-Case Western Reserve University Research Collaboration. Int J Tuberc Lung Dis 2000;4:1032–1040.

65. Mallory KF, Churchyard GJ, Kleinschmidt I, De Cock KM, Corbett EL. The impact of HIV infection on recurrence of tuberculosis in South African gold miners. Int J Tuberc Lung Dis 2000;4:455–462.

66. Mitchison DA, Nunn AJ. Influence of initial drug resistance on the response to short-course chemotherapy of pulmonary tuberculosis. Am Rev Respir Dis 1986;133:423–430.

68. Thomas A, Gopi PG, Santha T, et al. Predictors of relapse among pulmonary tuberculosis patients treated in a DOTS programme in South India. Int J Tuberc Lung Dis 2005;9:556–561.

69. Nunn AJ, Phillips PP, Mitchison DA. Timing of relapse in short-course chemotherapy trials for tuberculosis. Int J Tuberc Lung Dis 2010;14:241–242.

70. Watanabe Y, Murakami S, Oda M, et al. Treatment of bronchial stricture due to endobronchial tuberculosis. World J Surg 1997;21:480–487.



71. Iwamoto Y, Miyazawa T, Kurimoto N, et al. Interventional bronchoscopy in the management of airway stenosis due to tracheobronchial tuberculosis. Chest 2004;126:1344–1352.


72. Lim SY, Park HK, Jeon K, et al. Factors predicting outcome following airway stenting for post-tuberculosis tracheobronchial stenosis. Respirology 2011;16:959–964.


73. Cohen MD, Weber TR, Rao CC. Balloon dilatation of tracheal and bronchial stenosis. AJR Am J Roentgenol 1984;142:477–478.


74. Kim H. Rigid bronchoscopy for post-tuberculosis tracheobronchial stenosis. Tuberc Respir Dis (Seoul) 2023;86:245–250.




75. Chung FT, Chen HC, Chou CL, et al. An outcome analysis of self-expandable metallic stents in central airway obstruction: a cohort study. J Cardiothorac Surg 2011;6:46.




76. Dumon JF, Cavaliere S, Diaz-Jimenez JP, et al. Seven-year experience with the dumon prosthesis. J Bronchol 1996;3:6–10.

77. Lee KW, Im JG, Han JK, Kim TK, Park JH, Yeon KM. Tuberculous stenosis of the left main bronchus: results of treatment with balloons and metallic stents. J Vasc Interv Radiol 1999;10:352–358.


78. Low SY, Hsu A, Eng P. Interventional bronchoscopy for tuberculous tracheobronchial stenosis. Eur Respir J 2004;24:345–347.


79. Ryu YJ, Kim H, Yu CM, Choi JC, Kwon YS, Kwon OJ. Use of silicone stents for the management of post-tuberculosis tracheobronchial stenosis. Eur Respir J 2006;28:1029–1035.


80. Verma A, Park HY, Lim SY, et al. Posttuberculosis tracheobronchial stenosis: use of CT to optimize the time of silicone stent removal. Radiology 2012;263:562–568.


81. Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 2002;22:1395–1409.


82. Ittrich H, Bockhorn M, Klose H, Simon M. The diagnosis and treatment of hemoptysis. Dtsch Arztebl Int 2017;114:371–381.



83. Walker CM, Rosado-de-Christenson ML, Martínez-Jiménez S, Kunin JR, Wible BC. Bronchial arteries: anatomy, function, hypertrophy, and anomalies. Radiographics 2015;35:32–49.


84. Prutsky G, Domecq JP, Salazar CA, Accinelli R. Antifibrinolytic therapy to reduce haemoptysis from any cause. Cochrane Database Syst Rev 2016;11:CD008711.



85. Bellam BL, Dhibar DP, Suri V, et al. Efficacy of tranexamic acid in haemoptysis: a randomized, controlled pilot study. Pulm Pharmacol Ther 2016;40:80–83.


86. Wand O, Guber E, Guber A, Epstein Shochet G, Israeli-Shani L, Shitrit D. Inhaled tranexamic acid for hemoptysis treatment: a randomized controlled trial. Chest 2018;154:1379–1384.


87. Vaidya S, Tozer KR, Chen J. An overview of embolic agents. Semin Intervent Radiol 2008;25:204–215.



88. Ittrich H, Klose H, Adam G. Radiologic management of haemoptysis: diagnostic and interventional bronchial arterial embolisation. Rofo 2015;187:248–259.


89. Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014;189:576–585.



90. Martínez-García MÁ, de Gracia J, Vendrell Relat M, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J 2014;43:1357–1367.


91. Choi H, Lee H, Ra SW, et al. Developing a diagnostic bundle for bronchiectasis in South Korea: a modified delphi consensus study. Tuberc Respir Dis (Seoul) 2022;85:56–66.




92. Araújo D, Shteinberg M, Aliberti S, et al. The independent contribution of Pseudomonas aeruginosa infection to long-term clinical outcomes in bronchiectasis. Eur Respir J 2018;51:1701953.


93. Dicker AJ, Lonergan M, Keir HR, et al. The sputum microbiome and clinical outcomes in patients with bronchiectasis: a prospective observational study. Lancet Respir Med 2021;9:885–896.


94. Murray MP, Turnbull K, Macquarrie S, Hill AT. Assessing response to treatment of exacerbations of bronchiectasis in adults. Eur Respir J 2009;33:312–318.


95. Bedi P, Cartlidge MK, Zhang Y, et al. Feasibility of shortening intravenous antibiotic therapy for bronchiectasis based on bacterial load: a proof-of-concept randomised controlled trial. Eur Respir J 2021;58:2004388.


96. Choi JY. Exacerbation prevention and management of bronchiectasis. Tuberc Respir Dis (Seoul) 2023;86:183–195.




97. Kempker RR, Vashakidze S, Solomonia N, Dzidzikashvili N, Blumberg HM. Surgical treatment of drug-resistant tuberculosis. Lancet Infect Dis 2012;12:157–166.



98. World Health Oranization. WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva: World Health Organization, 2019.
99. Cadranel J, Philippe B, Hennequin C, et al. Voriconazole for chronic pulmonary aspergillosis: a prospective multicenter trial. Eur J Clin Microbiol Infect Dis 2012;31:3231–3239.




100. Judson MA, Stevens DA. The treatment of pulmonary aspergilloma. Curr Opin Investig Drugs 2001;2:1375–1377.

101. Camuset J, Nunes H, Dombret MC, et al. Treatment of chronic pulmonary aspergillosis by voriconazole in nonimmunocompromised patients. Chest 2007;131:1435–1441.


102. De Beule K, De Doncker P, Cauwenbergh G, et al. The treatment of aspergillosis and aspergilloma with itraconazole, clinical results of an open international study (1982–1987). Mycoses 1988;31:476–485.


103. Dupont B. Itraconazole therapy in aspergillosis: study in 49 patients. J Am Acad Dermatol 1990;23(3 Pt 2):607–614.


104. Jain LR, Denning DW. The efficacy and tolerability of voriconazole in the treatment of chronic cavitary pulmonary aspergillosis. J Infect 2006;52:e133–e137.


105. Schweer KE, Wittersheim M, Bangard C, Frank KF, Cornely OA. Chronic pulmonary Aspergillosis. Four exemplary clinical cases and literature overview. Dtsch Med Wochenschr 2014;139:2242–2247German.
106. Quan DH, Kwong AJ, Hansbro PM, Britton WJ. No smoke without fire: the impact of cigarette smoking on the immune control of tuberculosis. Eur Respir Rev 2022;31:210252.



107. Xing Z, Sun T, Janssens JP, et al. Airflow obstruction and small airway dysfunction following pulmonary tuberculosis: a cross-sectional survey. Thorax 2023;78:274–280.



108. Nightingale R, Chinoko B, Lesosky M, et al. Respiratory symptoms and lung function in patients treated for pulmonary tuberculosis in Malawi: a prospective cohort study. Thorax 2022;77:1131–1139.



109. Leung CC, Yew WW, Chan CK, et al. Smoking adversely affects treatment response, outcome and relapse in tuberculosis. Eur Respir J 2015;45:738–745.


110. Siddharthan T, Gupte A, Barnes PJ. Chronic obstructive pulmonary disease endotypes in low- and middle-income country settings: precision medicine for all. Am J Respir Crit Care Med 2020;202:171–172.



111. Stolz D, Mkorombindo T, Schumann DM, et al. Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet 2022;400:921–972.


112. Kim CJ, Yoon HK, Park MJ, et al. Inhaled indacaterol for the treatment of COPD patients with destroyed lung by tuberculosis and moderate-to-severe airflow limitation: results from the randomized INFINITY study. Int J Chron Obstruct Pulmon Dis 2017;12:1589–1596.




113. Yum HK, Park IN. Effect of inhaled tiotropium on spirometric parameters in patients with tuberculous destroyed lung. Tuberc Respir Dis (Seoul) 2014;77:167–171.



114. Miravitlles M, Auladell-Rispau A, Monteagudo M, et al. Systematic review on long-term adverse effects of inhaled corticosteroids in the treatment of COPD. Eur Respir Rev 2021;30:210075.



115. Jo YS. Long-term outcome of chronic obstructive pulmonary disease: a review. Tuberc Respir Dis (Seoul) 2022;85:289–301.




116. Huang TM, Kuo KC, Wang YH, et al.; On the behalf of Taiwan Clinical Trial Consortium for Respiratory Diseases (TCORE). Risk of active tuberculosis among COPD patients treated with fixed combinations of long-acting beta2 agonists and inhaled corticosteroids. BMC Infect Dis 2020;20:706.


118. Arnold MT, Dolezal BA, Cooper CB. Pulmonary rehabilitation for chronic obstructive pulmonary disease: highly effective but often overlooked. Tuberc Respir Dis (Seoul) 2020;83:257–267.




119. Vogiatzis I, Rochester CL, Spruit MA, Troosters T, Clini EM, American Thoracic Society/European Respiratory Society Task Force on Policy in Pulmonary Rehabilitation. Increasing implementation and delivery of pulmonary rehabilitation: key messages from the new ATS/ERS policy statement. Eur Respir J 2016;47:1336–1341.


120. Migliori GB, Marx FM, Ambrosino N, et al. Clinical standards for the assessment, management and rehabilitation of post-TB lung disease. Int J Tuberc Lung Dis 2021;25:797–813.


121. Tsuboi T, Ohi M, Chin K, et al. Ventilatory support during exercise in patients with pulmonary tuberculosis sequelae. Chest 1997;112:1000–1007.


122. Ando M, Mori A, Esaki H, et al. The effect of pulmonary rehabilitation in patients with post-tuberculosis lung disorder. Chest 2003;123:1988–1995.


123. Singh SK, Naaraayan A, Acharya P, Menon B, Bansal V, Jesmajian S. Pulmonary rehabilitation in patients with chronic lung impairment from pulmonary tuberculosis. Cureus 2018;10:e3664.



124. Visca D, Zampogna E, Sotgiu G, et al. Pulmonary rehabilitation is effective in patients with tuberculosis pulmonary sequelae. Eur Respir J 2019;53:1802184.


125. Budweiser S, Moertl M, Jörres RA, Windisch W, Heinemann F, Pfeifer M. Respiratory muscle training in restrictive thoracic disease: a randomized controlled trial. Arch Phys Med Rehabil 2006;87:1559–65.


126. Muñoz-Torrico M, Rendon A, Centis R, et al. Is there a rationale for pulmonary rehabilitation following successful chemotherapy for tuberculosis? J Bras Pneumol 2016;42:374–385.



127. Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017;50:1700629.


128. Alene KA, Clements ACA, McBryde ES, et al. Mental health disorders, social stressors, and health-related quality of life in patients with multidrug-resistant tuberculosis: a systematic review and meta-analysis. J Infect 2018;77:357–367.


-
METRICS

-
- 0 Crossref
- 0 Scopus
- 687 View
- 297 Download
- Related articles
-
Long-term renal outcomes of patients with non-proliferative lupus nephritis2023 September;38(5)
Nutritional management in patients with chronic kidney disease2020 November;35(6)
Diagnosis and treatment of cystic lung disease2017 March;32(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


