 |
 |
| Korean J Intern Med > Volume 38(6); 2023 > Article |
|
Abstract
Lung cancer is a dismal disease as a leading cause of overall cancer death, but the development of immune checkpoint inhibitors (ICIs) in driver gene mutation negative metastatic non-small cell lung cancer (NSCLC) is changing the paradigm of lung cancer treatment. Recently, ICIs are expanding their treatment area to early-stage NSCLC and ICIs have also changed their treatment strategies of such patients. And it is important to appropriately select patients with resectable early-stage lung cancer through a multidisciplinary team approach and decrease the tumor relapse rate in the ICIs era. In this review article, we discuss the recently released neoadjuvant and adjuvant data of ICIs, their treatment rationale, and unmet needs in the treatment of early-stage NSCLC.
Since immune checkpoint inhibitors (ICIs) have changed the treatment paradigm of metastatic non-small cell lung cancer (NSCLC) without targetable molecular driver gene mutation(s), they are gradually expanding their treatment area to early-stage NSCLC. In the PACIFIC trial, 1-year consolidation treatment with durvalumab after concurrent chemoradiation therapy (CCRT) in patients with inoperable stage III NSCLC proved not only to prolong progression-free survival (PFS) but also to prolong overall survival (OS) [1]. As a result, durvalumab treatment has now become the standard treatment for inoperable stage III NSCLC after CCRT. Recent several clinical trials using ICIs to address the unmet need to improve resectability and reduce recurrence in operable stage I, II, and III patients have reported good results. Neoadjuvant chemotherapy in NSCLC has traditionally been conducted using cytotoxic chemotherapy, but its impact on clinical efficacy has remained modest [2]. As clinical research results using ICIs and targeted therapies have been reported, ICIs and targeted therapies have recently received a lot of attention in early-stage NSCLC [3ŌĆō7].
In particular, the combination regimen of ICIs with cytotoxic chemotherapy has gained attention in neoadjuvant treatment [8ŌĆō10]. The introduction of ICIs has significantly increased pathologic complete response (pCR) rates in neoadjuvant treatment, which were previously only 4% with cytotoxic agents alone, now reaching 20ŌĆō25% [11ŌĆō13]. Adjuvant cytotoxic chemotherapy for the elimination of residual lesions showed a modest improvement in lung cancer survival rate of about 5% compared to placebo, whereas adjuvant treatment using ICI has recently shown more promising results.
The present review aims to summarize the efficacy, rationale, and concerns of the neoadjuvant and adjuvant therapy based on ICIs in early-stage NSCLC, focusing on the biological rationales and to discuss the role of potential predictive biomarkers and the unmet need of current treatment strategies.
Neoadjuvant chemotherapy refers to the administration of anticancer treatment before surgery. The primary objective of neoadjuvant chemotherapy is to reduce tumor burden to facilitate complete resection (R0 resection) and control micro metastasis while also providing an opportunity to pre-validate effective anticancer agents. However, careful decision-making is necessary because surgery might not be proceeded due to disease progression, side effects during neoadjuvant chemotherapy, and the increased complexity of surgical procedures. A meta-analysis conducted by the NSCLC Meta-Analysis Collaborative Group involving 15 randomized clinical trials demonstrated an absolute 5% survival benefit at 5 years with neoadjuvant chemotherapy [2]. Although it has not received attention due to low clinical benefits, it has recently received attention as encouraging results have been reported in clinical trials of neoadjuvant treatment using ICIs [3]. Due to its nature, neoadjuvant clinical trials require a surrogate marker that can indirectly predict such OS benefit, as it takes 10 years or more of time and a lot of costs to prove the OS benefit [14]. The utility of neoadjuvant chemotherapy in NSCLC is primarily assessed based on achieving a pCR defined as the absence of residual viable tumor or a major pathologic response (MPR) with residual viable tumor of 10% or less. In surgical tissues, the remaining cancer cell areas were classified in 10% increments [11,15,16]. When recurrence-free survival and OS were analyzed, 0% and 1ŌĆō10% groups, respectively, showed significantly different hazard ratios compared to other groups. Both pCR and MPR have been closely associated with reduced risk of recurrence after surgery and long-term survival [17ŌĆō19]. Traditional cytotoxic chemotherapy alone in NSCLC has been reported to yield low pCR rates [11]. Most of neoadjuvant randomized controlled trials (RCTs) based on ICIs with cytotoxic chemotherapy have defined pCR and MPR as primary or secondary outcomes [12,13,20].
Neoadjuvant chemotherapy utilizing ICIs has shown encouraging outcomes in NSCLC. Clinical trials based on nivolumab, atezolizumab, or pembrolizumab have reported similar results, showing approximately 20ŌĆō60% pCR rates and 30ŌĆō80% MPR rates (Table 1) [8,10,12,13,20]. The phase III CheckMate 816 study was designed to demonstrate the clinical efficacy of three cycles of nivolumab plus chemotherapy over chemotherapy only in patients with resectable stage IB to IIIA NSCLC. Compared to chemotherapy alone, combination therapy showed prolonged event free survival (31.6 vs. 20.8 mo, hazard ratio [HR] 0.63, p = 0.005). Significantly higher pCR was observed in nivolumab plus chemotherapy over chemotherapy alone (24.0 vs. 2.2%, p < 0.001). The percentage of MPR was 36.9% with nivolumab plus chemotherapy and 8.9% with chemotherapy alone (OR = 5.70, 95% confidence interval [CI] 3.16ŌĆō10.26). In an exploratory subset analysis, circulating tumor DNA (ctDNA) clearance was more frequent with nivolumab plus chemotherapy versus chemotherapy and appeared to be associated with pCR. This means that neoadjuvant nivolumab plus chemotherapy further reduces the burden of minimal residual disease. Recently, many clinical trials have been conducted on ctDNA as a surrogate marker for minor residual disease after surgical resection.
Subgroup analyses have identified programmed death ligand 1 (PD-L1) expression, mutation gene status, age, and smoking history as potential factors influencing efficacy. A notable finding is that even when PD-L1 is expressed at Ōēź 1%, it demonstrates clinical benefits compared to the 0% group. There is still concern regarding prognostic difference based on high PD-L1 expression group (Ōēź 50%) versus low PD-L1 expression group (1ŌĆō49%). Mutation status such as absence of target genes like EGFR [8,10] and younger age have been found to be favorable prognostic factors. Some reports have suggested that current and former smokers have better outcomes than never-smokers. However, there are no distinct differences based on histology type or the type of cytotoxic chemotherapy. The impact of lymph node status and tumor stage on neoadjuvant chemotherapy response in NSCLC remains a concern, showing variable results [12,13,20].
The advancement of ICIs has introduced a multitude of treatment options, creating a decision difficulty in clinical fields. For example, for stage III NSCLC, there are many treatment options, such as neoadjuvant ICI therapy followed by surgery, adjuvant ICI therapy after surgery, and ICI therapy following concurrent chemoradiotherapy [21ŌĆō23]. Factors such as multidisciplinary team decisions, patient conditions, country-specific ICI approvals, and hospital-specific circumstances can affect treatment selection. Since RCT that directly compares them has not been reported yet, the contents described in this review should be considered as hypotheses or expert opinions. Further basic research and RCTs are needed to determine the optimal neoadjuvant and adjuvant treatment based on ICIs in NSCLC.
In cytotoxic chemotherapy-based regimens, there has been no observed difference in prognosis between neoadjuvant and adjuvant treatments [2,24]. However, in ICI based therapies, there is a potential disparity due to the presence or absence of targetable cancer cells and cancer antigens by immune cells in neoadjuvant and adjuvant settings.
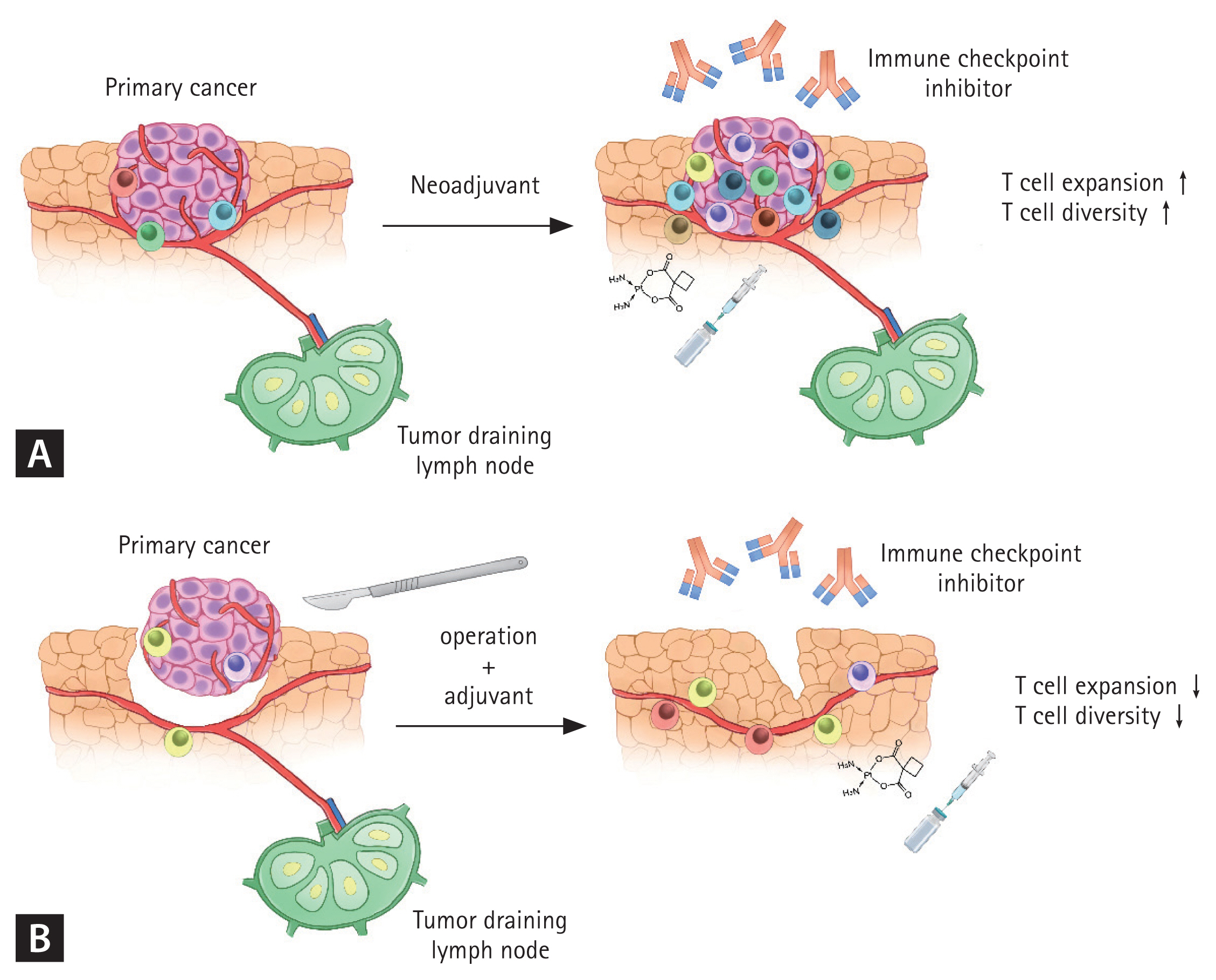
In neoadjuvant treatment, residual cancer cells including the primary tumor site and tumor-draining lymph nodes (TDLNs) remain. However, surgical resection in NSCLC involves the removal of the primary tumor and comprehensive lymph node dissection, which can result in direct reduction of tumor antigens that can be targeted by various immune cells. Recently, it has been shown that TDLNs have a positive effect on cytotoxic lymphocyte expansion and diversity of T cell receptor (TCR) [25ŌĆō28]. In neoadjuvant treatment where residual cancer cells remain in the body, more diverse T cell responses can be generated than those in an adjuvant setting [28ŌĆō30]. Adjuvant immunotherapy can also restore antitumor immunity that might have been compromised by surgery [31]. Despite this, some adjuvant RCTs have not demonstrated a significant improvement in OS. A study using mouse models has suggested the superiority of neoadjuvant over adjuvant therapy [32]. Although there is no directly comparable RCTs, neoadjuvant treatment which allows immune cells to recognize remaining tumor cells and TDLNs appears to be more a favorable perioperative setting for lymphocyte expansion and diversity of TCR than adjuvant treatment (Fig. 1).
ICIs appear to be more advantageous in neoadjuvant treatment as evidenced by OS data from adjuvant RCTs [12,13,20]. Two representative RCTs that used ICIs as adjuvant treatment, namely IMpower010 (atezolizumab) and PEARLS/KEYNOTE-091 (pembrolizumab), showed significant benefits in the primary endpoint of disease-free survival. Both the IMPOWER 010 study and the PEARLS/KEYNOTE 091 study met their primary endpoint of disease-free survival with HRs of 0.81 (95% CI, 0.67ŌĆō0.99) and 0.76 (95% CI, 0.63ŌĆō0.91), respectively (Table 2). Although there is a limitation in the interpretation that the OS data have not yet matured, neither study demonstrated significant benefits in the secondary outcome of OS [33,34]. Based on a sub-analysis of the IMpower010 study, it could be inferred that OS benefits were observed only in a specific subgroup with PD-L1 expression of 50% or higher and negative EGFR/ALK status [35]. On the other hand, the PEARS/KEYNOTE-091 study demonstrated prolongation of disease-free survival regardless of PD-L1 expression lesions, and according to the guideline, pembrolizumab is recommended for PD-L1-unselected patients with stage IB to IIIA NSCLC. In an adjuvant setting, a clearer definition of the patient population or additional studies such as perioperative treatment (neoadjuvant + adjuvant) might be necessary.
There are several concerns associated with neoadjuvant therapy using ICIs: 1) potential inability to proceed with surgery due to treatment-related side effects and cancer progression during neoadjuvant therapy; 2) neoadjuvant therapy might complicate surgical procedure due to development of fibrosis, scar changes, or adhesions. There are also special risks associated with ICIs therapy, such as hyper-progressive disease (HPD), pseudo-progression, and immune-related adverse events (irAEs).
Based on analysis of CheckMate 816, in a group receiving ICIs with cytotoxic chemotherapy (ICI group), 83% of patients were able to undergo surgery, while in the cytotoxic chemotherapy alone group (cytotoxic alone group), 75% of patients were able to undergo surgery [12]. The incidence of delayed surgery for various reasons was 21% in the ICI group and 18% in the cytotoxic alone group. These findings suggest that the use of ICIs in neoadjuvant treatment does not significantly increase the difficulty of surgery. It allows a higher proportion of patients to undergo surgery. Particularly in stage IIIA patients, the ICI group showed a 20% higher rate of undergoing lobectomy and a 13% lower rate of undergoing pneumonectomy compared to the cytotoxic alone group. This indicates a significant downstaging effect associated with the use of ICIs.
The NADIM study, similar to CheckMate 816, demonstrated that neoadjuvant ICI therapy did not have a negative impact on surgery-related outcomes. After excluding those who refused surgery, 41 (93.2%) of 44 patients underwent surgery. In this study, neither PD-L1 expression nor tumor mutation burden predicted long-term survival, while a significant association between ctDNA levels after neoadjuvant chemoimmunotherapy and survival benefits was shown. No patient experienced treatment-related adverse events that delayed surgery or resulted in death. Additionally, there was no intraoperative or in-hospital mortality (30 or 90 days after operation) [10].
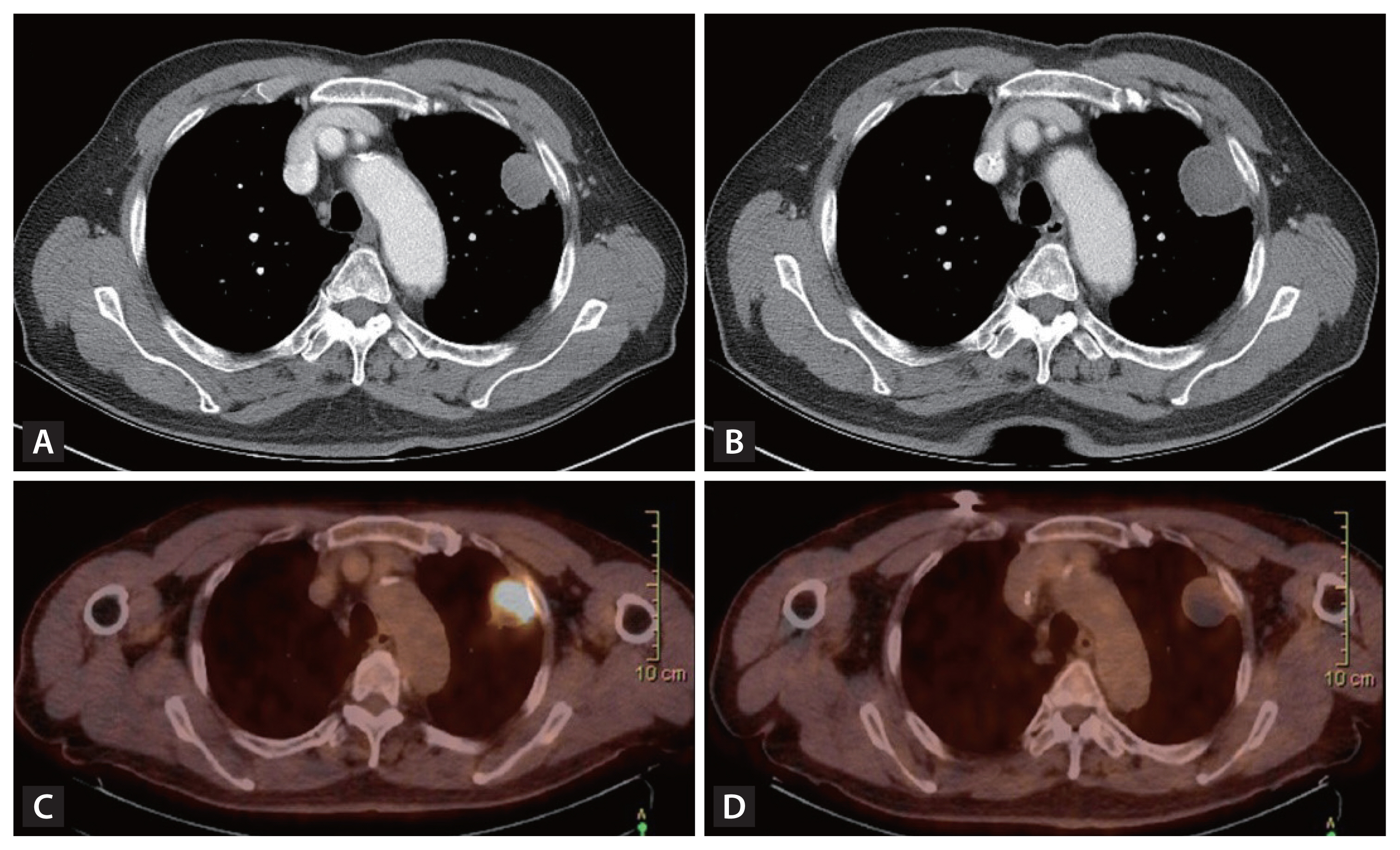
Furthermore, even in cases where chest computed tomography (CT) evaluation after neoadjuvant treatment showed tumor progression, the nature of ICIs therapy (pseudo-progression) resulted in minimal or absence of cancer cells in actual surgical specimens [3,36,37]. In the neoadjuvant setting, the discrimination of true progression and pseudo-progression is particularly critical, to not preclude curative surgery. This highlights the need for additional imaging tools such as chest magnetic resonance imaging or positron emission tomography in response assessment as well as the importance of considering surgery more actively even for cases showing tumor growth (Fig. 2) [38ŌĆō40].
Currently, there is no definitive evidence regarding the extent of surgical difficulty when using ICIs in neoadjuvant treatment. However, from surgeonsŌĆÖ perspective, achieving complete resection (R0 resection) is the most important thing in decision-making. The use of ICIs in a neoadjuvant setting has already shown significant benefits in terms of achieving pCR or MPR [8ŌĆō10]. Even in cases where complete resection appears challenging based on chest CT imaging, there have been instances of achieving pCR in surgical specimens, which indicates that ICIs based neoadjuvant therapy is highly advantageous. Nevertheless, further research about ICIs is needed on factors such as surgical difficulty, postoperative management, and complications [41ŌĆō43].
When using ICIs, there are always concerns regarding HPD and irAEs [44,45]. In several RCTs, the incidence of all-grade irAEs was around 20%. However, the occurrence of grade IV irAEs that require surgical delay or render surgery impossible is very low, ranging from 1ŌĆō2%. Additionally, it has been observed that the length of hospital stay following surgery does not significantly increase with the use of ICIs [12,13,20]. Furthermore, while there might be concerns about HPD with ICIs monotherapy, the risk is very low when ICIs are combined with cytotoxic chemotherapy [46].
Another issue of the neoadjuvant ICI therapy is that some patients with MPR showed recur, and even patients with pCR also had recur, so attempts to reduce the number of recurring patients after such surgery are currently underway. As part of this, perioperative treatment, which treats ICI before and after surgery, is attracting attention.
As a strategy to compensate for the pros and cons of neoadjuvant and adjuvant treatments, perioperative immunotherapy plus cytotoxic chemotherapy is a trend of clinical trial that has recently been newly attempted. Of course, there may be a patient group for which only neoadjuvant treatment is indicated, and there may be patients for whom only adjuvant treatment is indicated. For example, in the case of stage IB or II, if immediate surgery is possible, adjuvant immunotherapy can be performed immediately after surgery without neoadjuvant treatment. When surgery was performed after neoadjuvant immuno-cytotoxic chemotherapy, the relapse rate is high, especially in stage II and III patients, so it is difficult to expect long term survival of patients with neoadjuvant treatment alone. In particular, perioperative strategies were mainly clinically conducted in II or IIIA/B because the stage in which complete resection is difficult at the time of diagnosis is the most appropriate indication. Recently, the phase II NADIM II trial and the phase III Keynote 671 trial reported the usefulness of the perioperative treatment strategy in patients with early-stage NSCLC [13,20]. In NADIM II trial, patients with resectable stage IIIA or IIIB NSCLC were enrolled and received neoadjuvant nivolumab plus platinum-based chemotherapy (experimental group) or chemotherapy alone (control group), followed by surgery. Patients in the nivolumab plus chemotherapy group who had R0 resections received adjuvant treatment with nivolumab for 6 months. A pCR occurred in 37% of the patients in the experimental group and in 7% in the control group. OS at 24 months was 85.0% in the experimental group and 63.6% in the control group. In Keynote-671 trial, Patients with resectable stage II, IIIA, or IIIB (N2 stage) NSCLC were enrolled and received neoadjuvant pembrolizumab plus cisplatin-based chemotherapy or chemotherapy alone for 4 cycles, followed by surgery and adjuvant pembrolizumab or placebo for up to 13 cycles. The primary endpoint of event-free survival demonstrated a clinically meaningful improvement with the Pembrolizumab group, with an HR of 0.58 (95% CI, 0.46ŌĆō0.72, p < 0.00001). A pCR occurred in 18.1% of the pembrolizumab group and 4.0% of the placebo group. Although the absolute value of pCR in both clinical trials is different, it is a higher value compared to the pCR value seen in conventional cytotoxic chemotherapy (median, 4%) [11]. Although the OS data has not been matured yet, the interim analysis data showed a promising date, so the final analysis result is expected in the future.
Durvalumab maintenance treatment after CCRT in unresectable stage III NSCLC proved the benefit of PFS and OS (PACIFIC trial), but the issue of resectability in stage III NSCLC is still an unmet need [1]. Although the multidisciplinary team approach determines the direction of treatment at the N2 stage, in some cases, there is no consensus on the appropriate treatment at the N2 stage. Because the decision regarding resectability has been mostly driven by poor survival outcomes rather than the feasibility of surgery. Since the application of ICIs plus cytotoxic chemotherapy improved the resectability of surgery and the introduction of adjuvant immunotherapy reduced the relapse rate, it has become very important to select appropriate patients through a multidisciplinary team approach.
Neoadjuvant treatment based on ICIs in NSCLC is expected to rapidly expand in clinical practice. However, several controversial issues need to be addressed. Various RCTs are currently underway to address these issues (Table 3). The main issue is related to regimen (= combination drug selection). Although this review article mainly discussed ICIs with platinum based cytotoxic chemotherapy, there are various drug options depending on patient characteristics, including ICIs monotherapy, ICIs with other ICIs, or ICIs combined with targeted therapy (especially anti-angiogenesis agents) [4,47,48]. This issue is ultimately related to the optimal selection of patient populations, which is associated with biomarkers. Targeted therapy seems to be more beneficial than using ICIs in neoadjuvant/adjuvant settings when there are common mutation genes such as EGFR, ALK, ROS-1, and KRAS. For patients without these common mutation genes, a predictive biomarker is necessary to determine the optimal patient population. Currently, known common biomarkers include PD-L1 expression [12,13,20], tumor mutational burden (TMB) [3], ctDNA [49], microsatellite instability-high (MSI-H), and mismatch repair deficiency [50ŌĆō52].
While there might be variations in results across different RCTs, it is generally observed that PD-L1, TMB, and MSI-H have a positive correlation with response rates, while ctDNA shows a negative correlation. In particular, it has been shown that ctDNA detected before and after surgery is closely associated with relapse [53,54]. There is a potential benefit for patients who have ctDNA detected after surgery to maintain adjuvant ICIs treatment following neoadjuvant ICIs treatment [55]. Some studies are currently investigating the use of specific genes such as RREB1 and SSPO mutations or employing a peripheral T cell expansion score. However, further research is needed for their practical application in clinical settings [56].
Another issue in perioperative treatment is the optimal cycle issue in neoadjuvant treatment. The issue with 3 cycles or 4 cycles is that treatment of less than 3 cycles is difficult to obtain the maximum therapeutic effect, whereas treatment of a longer period may miss the surgical timing due to disease progression. And most of the current clinical studies are conducting adjuvant ICIs treatment for 1 year, and the answer to whether a 1-year period is appropriate should wait for long-term results of clinical trials and real-world data. In addition, clinical trials are underway to determine whether to perform adjuvant immunotherapy by predicting molecular residual disease status through ctDNA, and appropriate target patients for optimal adjuvant immunotherapy can be determined according to the results.
The development of ICIs has led to rapid advancements in the treatment of lung cancer. Initially, ICIs were predominantly utilized in palliative settings for advanced stage IV NSCLC or extensive-stage small cell lung cancer. Recently, ICIs have expanded their application to early-stage lung cancer, including neoadjuvant and adjuvant approaches. Conventional cytotoxic chemotherapy has shown limited efficacy in early-stage lung cancer. However, it has been demonstrated that ICIs can significantly improve rates of pCR and MPR. And adjuvant immunotherapy demonstrated a reduction in the risk of disease recurrence or death. In conclusion, it is important to appropriately select patients with resectable early-stage lung cancer through a multidisciplinary team approach, and it is increasingly important to establish a treatment strategy based on biomarkers that can improve survival by reducing relapse through adjuvant immunotherapy after surgery.
Notes
CRedit authorship contributions
Jae Kyeom Sim: resources, validation, writing - original draft; Juwhan Choi: resources, formal analysis, writing - original draft, visualization; Sung Yong Lee: conceptualization, validation, writing - review & editing, supervision, project administration, funding acquisition
Figure┬Ā1
Mechanism of potential advantage of the neoadjuvant immunotherapy over the adjuvant immunotherapy. Preoperative primary tumor has diverse and abundant amount of tumor antigens. Immune checkpoint inhibitor restores immune response to tumor antigens resulting in increased T-cell diversity and expansion of T-cell clones in primary tumor. Immune checkpoint inhibitor also enhances tumor antigen presentation by antigen presenting cell and activation of T-cells in tumor-draining lymph node (TDLN) (A). When the primary tumor and lymph nodes, including TDLN, are surgically removed, both the quantity and variety of tumor antigens diminish. Additionally, the immune response within in the TDLN is lost. Consequently, the effectiveness of immune checkpoint inhibitor in eliciting a robust T cell response is decreased (B).
Figure┬Ā2
Discordance between radiologic and metabolic responses after neoadjuvant immunotherapy. A case of a 73-year-old male patient with non-small cell lung cancer (adenocarcinoma) treated with neoadjuvant immunotherapy and received left upper lobectomy and showed pathologic complete remission. (A) Chest computed tomography (CT) before immunotherapy. (B) Chest CT after immunotherapy in the same patient. The longest tumor diameter was increased from 3.2 cm to 3.7 cm. (C) Positron emission tomography-CT (PET-CT) before immunotherapy in the same patient. (D) PET-CT after immunotherapy in the same patient. The standardized uptake value was decreased from 14.83 to 2.26.
Table┬Ā1
Clinical trials of neoadjuvant/perioperative immunotherapy in NSCLC
| Study | Stage | Study design | Patients numbers | Regimens | MPR (%) | pCR (%) | R0 resection (%) |
|---|---|---|---|---|---|---|---|
| Neoadjuvant trial | |||||||
| ŌĆāCheckMate816 (NCT02998528) [1] | IB to IIIA | Phase III | 179 | Nivo + platinum doublet 3 cycles | 66 (36.8) | 43 (24.0) | 124 (69.3) |
| ŌĆāNCT02716038 [8] | IB to IIIA | Phase II | 30 | Atezo + platinum doublet 4 cycles | 17 (56.7) | 10 (33.3) | 26 (86.7) |
| ŌĆāNEOSTAR (NCT03158129)-Arm A [4] | I to IIIA | Phase II | 23 | Nivo 3 cycles | 5 (21.7) | 2 (8.7) | 23 (100.0) |
| ŌĆāNEOSTAR (NCT03158129)-Arm B [4] | I to IIIA | Phase II | 21 | Nivo 3 cycles + ipilimumab 1 cycle | 8 (38.1) | 6 (28.6) | 21 (100.0) |
| ŌĆāNEOSTAR (NCT03158129)-Arm C [5] | IB to IIIA | Phase II | 22 | Nivo 3 cycles + cytotoxic chemotherapy 3 cycles | 7 (32.1) | 4 (18.2) | 20 (90.9) |
| ŌĆāNEOSTAR (NCT03158129)-Arm D [5] | IB to IIIA | Phase II | 22 |
Nivo 3 cycles + ipilimumab 1 cycle + cytotoxic chemotherapy 3 cycles |
11 (50.0) | 4 (18.2) | 19 (95.0) |
| Perioperative (Neoadjuvant + adjuvant) trial | |||||||
| ŌĆāNADIM (NCT03081689) [10] | IIIA | Phase II | 46 | Nivo + platinum doublet 3 cycles ŌĆō Nivo (adj) 12 months | 34 (82.9) | 26 (63.4) | 41 (89.1) |
| ŌĆāKEYNOTE 671 (NCT03425643) [13] | II to IIIB | Phase III | 397 | Pembro + platinum doublet 4 cycles ŌĆō Pembro (adj) 13 cycles | 120 (30.2) | 72 (18.1) | 299 (75.3) |
| ŌĆāNADIM II (NCT03838159) [20] | IIIA to | Phase II | 57 | Nivo + platinum doublet 3 cycles ŌĆō Nivo (adj) 6 months | 30 (52.6) | 21 (36.8) | 50 (87.7) |
| ŌĆāSAKK 16/14 [9] | IIIA | Phase II | 55 | Cisplatin/docetaxel 3 cycles -> Durvalumab 2cycles ŌĆō Durvalumab(adj) 12 months | 34 (61.8) | 10 (18.2) | 51 (92.7) |
Table┬Ā2
Representative adjuvant clinical trials of immunotherapy in NSCLC
| Study | Stage | Study design | Patients numbers | Regimens | DFS, HR (95% CI) | Follow-up period (mo) |
|---|---|---|---|---|---|---|
| IMpower010 [33] | IB to IIIA | Phase III | 1280 | Atezolizumab 16 cycles or 1 year | 0.81 (0.67ŌĆō0.99, p = 0.040) | 32.2 |
| PEARLS/KEYNOTE-091 [34] | IB to IIIA | Phase III | 1177 | Pembrolizumab 18 cycles | 0.76 (0.63ŌĆō0.91, p = 0.014) | 35 |
Table┬Ā3
Ongoing - clinical trials of neoadjuvant/perioperative immunotherapy in NSCLC
REFERENCES
1. Antonia SJ, Villegas A, Daniel D, et al.; PACIFIC Investigators. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342ŌĆō2350.


2. NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383:1561ŌĆō1571.



3. Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018;378:1976ŌĆō1986.


4. Cascone T, William WN Jr, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med 2021;27:504ŌĆō514.


5. Cascone T, Leung CH, Weissferdt A, et al. Neoadjuvant chemotherapy plus nivolumab with or without ipilimumab in operable non-small cell lung cancer: the phase 2 platform NEOSTAR trial. Nat Med 2023;29:593ŌĆō604.


6. Lv C, Fang W, Wu N, et al. Osimertinib as neoadjuvant therapy in patients with EGFR-mutant resectable stage IIŌĆōIIIB lung adenocarcinoma (NEOS): a multicenter, single-arm, open-label phase 2b trial. Lung Cancer 2023;178:151ŌĆō156.


7. Zhang C, Li SL, Nie Q, et al. Neoadjuvant crizotinib in resectable locally advanced non-small cell lung cancer with ALK rearrangement. J Thorac Oncol 2019;14:726ŌĆō731.


8. Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:786ŌĆō795.


9. Rothschild SI, Zippelius A, Eboulet EI, et al.; Swiss Group for Clinical Cancer Research (SAKK). SAKK 16/14: durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small-cell lung cancer-a multicenter single-arm phase II trial. J Clin Oncol 2021;39:2872ŌĆō2880.

10. Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:1413ŌĆō1422.


11. Hellmann MD, Chaft JE, William WN Jr, et al.; University of Texas MD Anderson Lung Cancer Collaborative Group. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42ŌĆōe50.



12. Forde PM, Spicer J, Lu S, et al.; CheckMate 816 Investigators. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med 2022;386:1973ŌĆō1985.


13. Wakelee H, Liberman M, Kato T, et al.; KEYNOTE-671 Investigators. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med 2023;389:491ŌĆō503.


14. Weissferdt A, Pataer A, Vaporciyan AA, et al. Agreement on major pathological response in NSCLC patients receiving neoadjuvant chemotherapy. Clin Lung Cancer 2020;21:341ŌĆō348.



15. Pataer A, Weissferdt A, Correa AM, et al. Major pathologic response and prognostic score predict survival in patients with lung cancer receiving neoadjuvant chemotherapy. JTO Clin Res Rep 2022;3:100420.



16. Travis WD, Dacic S, Wistuba I, et al. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol 2020;15:709ŌĆō740.



17. Mouillet G, Monnet E, Milleron B, et al.; Intergroupe Francophone de Canc├®rologie Thoracique (IFCT). Pathologic complete response to preoperative chemotherapy predicts cure in early-stage non-small-cell lung cancer: combined analysis of two IFCT randomized trials. J Thorac Oncol 2012;7:841ŌĆō849.


18. Pataer A, Kalhor N, Correa AM, et al.; University of Texas M. D. Anderson Lung Cancer Collaborative Research Group. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2012;7:825ŌĆō832.



19. Rosner S, Liu C, Forde PM, Hu C. Association of pathologic complete response and long-term survival outcomes among patients treated with neoadjuvant chemotherapy or chemoradiotherapy for NSCLC: a meta-analysis. JTO Clin Res Rep 2022;3:100384.



20. Provencio M, Nadal E, Gonz├Īlez-Larriba JL, et al. Perioperative nivolumab and chemotherapy in stage III non-small-cell lung cancer. N Engl J Med 2023;389:504ŌĆō513.

21. Ortega-Franco A, Calvo V, Franco F, Provencio M, Califano R. Integrating immune checkpoint inhibitors and targeted therapies in the treatment of early stage non-small cell lung cancer: a narrative review. Transl Lung Cancer Res 2020;9:2656ŌĆō2673.



22. Lim SW, Ahn MJ. Current status of immune checkpoint inhibitors in treatment of non-small cell lung cancer. Korean J Intern Med 2019;34:50ŌĆō59.




23. Lim JU. Update on adjuvant treatment in resectable non-small cell lung cancer and potential biomarkers predicting postoperative relapse. Tuberc Respir Dis (Seoul) 2023;86:14ŌĆō22.




24. Pignon JP, Tribodet H, Scagliotti GV, et al.; LACE Collaborative Group. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552ŌĆō3559.


25. Jung CY, Antonia SJ. Tumor immunology and immune checkpoint inhibitors in non-small cell lung cancer. Tuberc Respir Dis (Seoul) 2018;81:29ŌĆō41.




26. Fransen MF, Schoonderwoerd M, Knopf P, et al. Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. JCI Insight 2018;3:e124507.



27. Francis DM, Manspeaker MP, Schudel A, et al. Blockade of immune checkpoints in lymph nodes through locoregional delivery augments cancer immunotherapy. Sci Transl Med 2020;12:eaay3575.



28. du Bois H, Heim TA, Lund AW. Tumor-draining lymph nodes: at the crossroads of metastasis and immunity. Sci Immunol 2021;6:eabg3551.


29. Gaudreau PO, Negrao MV, Mitchell KG, et al. Neoadjuvant chemotherapy increases cytotoxic T cell, tissue resident memory T cell, and B cell infiltration in resectable NSCLC. J Thorac Oncol 2021;16:127ŌĆō139.



30. Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 2020;367:eaax0182.



31. Matzner P, Sandbank E, Neeman E, Zmora O, Gottumukkala V, Ben-Eliyahu S. Harnessing cancer immunotherapy during the unexploited immediate perioperative period. Nat Rev Clin Oncol 2020;17:313ŌĆō326.



32. Liu J, Blake SJ, Yong MC, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov 2016;6:1382ŌĆō1399.



33. Felip E, Altorki N, Zhou C, et al.; IMpower010 Investigators. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IBŌĆōIIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021;398:1344ŌĆō1357.


34. OŌĆÖBrien M, Paz-Ares L, Marreaud S, et al.; EORTC-1416-LCG/ETOP 8-15 ŌĆō PEARLS/KEYNOTE-091 Investigators. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IBŌĆōIIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol 2022;23:1274ŌĆō1286.


35. Felip E, Altorki N, Zhou C, et al. Overall survival with adjuvant atezolizumab after chemotherapy in resected stage IIŌĆōIIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase III trial. Ann Oncol 2023;34:907ŌĆō919.


36. William WN Jr, Pataer A, Kalhor N, et al.; University of Texas M.D. Anderson Lung Cancer Collaborative Research Group. Computed tomography RECIST assessment of histopathologic response and prediction of survival in patients with resectable non-small-cell lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2013;8:222ŌĆō228.



37. Katz SI, Hammer M, Bagley SJ, et al. Radiologic pseudoprogression during anti-PD-1 therapy for advanced non-small cell lung cancer. J Thorac Oncol 2018;13:978ŌĆō986.


38. Bao X, Bian D, Yang X, et al. Multiparametric MRI for evaluation of pathological response to the neoadjuvant chemo-immunotherapy in resectable non-small-cell lung cancer. Eur Radiol 2023;Jun. 29. [Epub]. 10.1007/s00330-023-09813-8.



39. Lee HY, Lee HJ, Kim YT, et al. Value of combined interpretation of computed tomography response and positron emission tomography response for prediction of prognosis after neoadjuvant chemotherapy in non-small cell lung cancer. J Thorac Oncol 2010;5:497ŌĆō503.


40. Gao S, Li N, Gao S, et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol 2020;15:816ŌĆō826.


41. Wislez M, Mazieres J, Lavole A, et al. Neoadjuvant durvalumab for resectable non-small-cell lung cancer (NSCLC): results from a multicenter study (IFCT-1601 IONESCO). J Immunother Cancer 2022;10:e005636.



42. Bott MJ, Yang SC, Park BJ, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:269ŌĆō276.



43. Tong BC, Gu L, Wang X, et al. Perioperative outcomes of pulmonary resection after neoadjuvant pembrolizumab in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 2022;163:427ŌĆō436.


44. Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 2017;23:4242ŌĆō4250.




45. Choi J, Lee SY. Clinical characteristics and treatment of immune-related adverse events of immune checkpoint inhibitors. Immune Netw 2020;20:e9.




46. Matsuo N, Azuma K, Kojima T, et al. Comparative incidence of immune-related adverse events and hyperprogressive disease in patients with non-small cell lung cancer receiving immune checkpoint inhibitors with and without chemotherapy. Invest New Drugs 2021;39:1150ŌĆō1158.



47. Song Y, Fu Y, Xie Q, Zhu B, Wang J, Zhang B. Anti-angiogenic agents in combination with immune checkpoint inhibitors: a promising strategy for cancer treatment. Front Immunol 2020;11:1956s.



48. Reck M, Mok TSK, Nishio M, et al.; IMpower150 Study Group. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019;7:387ŌĆō401.


49. Provencio M, Serna-Blasco R, Nadal E, et al. Overall survival and biomarker analysis of neoadjuvant nivolumab plus chemotherapy in operable stage IIIA non-small-cell lung cancer (NADIM phase II trial). J Clin Oncol 2022;40:2924ŌĆō2933.



50. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509ŌĆō2520.


51. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409ŌĆō413.


52. Olivares-Hern├Īndez A, Del Barco Morillo E, Parra P├®rez C, et al. Influence of DNA mismatch repair (MMR) system in survival and response to immune checkpoint inhibitors (ICIs) in non-small cell lung cancer (NSCLC): retrospective analysis. Biomedicines 2022;10:360.



53. Hu W, Yang Y, Zhang L, et al. Post surgery circulating free tumor DNA is a predictive biomarker for relapse of lung cancer. Cancer Med 2017;6:962ŌĆō974.




54. Yue D, Liu W, Chen C, et al. Circulating tumor DNA predicts neoadjuvant immunotherapy efficacy and recurrence-free survival in surgical non-small cell lung cancer patients. Transl Lung Cancer Res 2022;11:263ŌĆō276.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



