INTRODUCTION
Gallbladder polyps (GBPs), characterized by protruding lesions in the mucosal lining of the gallbladder, are often incidentally detected on abdominal ultrasonography (US) [
1]. GBP are usually benign; however, their malignant potential makes them clinically significant [
2]. Although the reported malignancy rate is relatively low (3ŌĆō8%), gallbladder cancer (GBC) is highly lethal because it is often not diagnosed until at an advanced stage [
2,
3]. Some GBC cases can present as polyp-like lesions. However, abdominal US, commonly used for evaluating gallbladder diseases, has limited sensitivity for detecting early-stage GBC and cannot accurately distinguish between neoplastic and non-neoplastic polyps [
2,
4,
5]. Moreover, most GBP and GBC cases are asymptomatic or present only with nonspecific symptoms. The prevalence of GBP recently increased in Asian countries, including Korea [
2]. Therefore, it is crucial to identify and address modifiable risk factors for GBP to prevent further increases in its incidence and associated complications.
Hepatitis B virus (HBV) infection is a risk factor for GBP [
2,
6-
13]. Many epidemiological studies, including those in HBV infectionŌĆōendemic areas, reported that the presence of hepatitis B surface antigen (HBsAg) is significantly associated with an increased risk of GBP [
7-
13]. Several meta-analyses reported a positive association between the presence of HBsAg and GBP [
6]. However, other studies reported weak or null associations [
2,
14,
15]. Additionally, in a Korean study that evaluated the temporal changes in the prevalence and risk factors of GBP for approximately 10 years, HBsAg positivity was a risk factor in the early (2002ŌĆō2004) but not later (2010ŌĆō2012) period [
2]. A nationwide HBV vaccination program in Korea may contribute to reducing the prevalence of HBV infection, and the impact of HBV on GBP development may decline [
2,
6]. However, previous studies of HBsAg positivity and risk of GBP were based on data obtained many years ago, even 20 years ago [
2,
6-
14]. Therefore, we need to determine whether HBsAg remains an important predisposing factor for GBP in these days when the prevalence of this infection has decreased.
Regarding hepatitis C virus (HCV) infection, the presence of hepatitis C antibody (anti-HCV) is a well-established risk factor for cholelithiasis [
16,
17]; however, the effect of anti-HCV positivity on GBP development is unclear [
2,
9], as is whether HCV infection affects GBP risk.
Therefore, this study aimed to evaluate the association between HBV and HCV infections and GBP risk in a large sample of Korean adults. We sought to identify whether the presence of HBsAg, hepatitis B core antibody (HBcAb), and anti-HCV status is independently associated with GBP risk. Since differentiating GBP < 5 mm diameter from gallstone disease is difficult and most GBP < 5 mm are non-neoplastic [
4,
10], we determined GBP risk by considering clinically significant GBP Ōēź 5 mm or Ōēź 10 mm in addition to overall size (< 5, 5ŌĆō9, and Ōēź 10 mm).
METHODS
Study population
The Kangbuk Samsung Health Study comprised a group of adult Korean males and females aged Ōēź 18 years who underwent a comprehensive health check-up annually or biannually at a Kangbuk Samsung Hospital Total Healthcare Center clinic in Seoul or Suwon, South Korea, as previously described [
18,
19]. The study population comprised a subset of Kangbuk Samsung Health Study participants who underwent abdominal US screening between January 2011 and December 2016 (n = 419,058). According to the Industrial Safety and Health Law of Korea, employees must undergo health checkups annually or biannually. Approximately 80% of the individuals who participated in the screening program were employed by various companies or local government organizations (or were their spouses), and the remaining participants chose to volunteer for the program.
The exclusion criteria were as follows (
Fig. 1): (1) history of cholecystectomy; (2) history of malignancy, including GBC, hepatocellular carcinoma, and cholangiocarcinoma; (3) incomplete data on HBsAg, HBcAb, and anti-HCV; and (3) incomplete data on other demographic factors, including smoking status and alcohol consumption. After excluding 26,145 participants who met one or more exclusion criteria, the final sample comprised 392,913 participants.
This study was approved by the Institutional Review Board of the Kangbuk Samsung Hospital (no. KBSMC 2023-02-023), which waived the requirement for informed consent since the study utilized anonymized retrospective data collected during routine health screening procedures.
Measurements and definitions of variables
Standardized self-administered questionnaires were used to obtain demographic characteristics, health-related behaviors, and medical histories, whereas trained staff measured anthropometry, blood pressure, and serum biochemical parameters during health examinations [
18,
19]. Smoking status was classified as never, former, or current, while the frequency of moderate or vigorous physical activity per week was evaluated. The Korean version of the International Physical Activity Questionnaire was used to estimate physical activity levels, which were converted into a metabolic equivalent (MET) score and grouped into three categories: inactive, minimally active, and health-enhancing physical active (HEPA) [
20]. HEPA level was defined as meeting one of the following criteria: engaging in physical activity that consumes > 1,500 MET minutes per week, which includes at least three days of vigorous activity; or engaging in physical activity that consumes > 3,000 MET minutes per week [
20].
Blood samples were collected from the participants after a minimum fasting period of 10 hours, and blood tests included viral hepatitis indices, including HBsAg, HBcAb, and anti-HCV, liver enzymes, lipid profiles, high-sensitivity C-reactive protein, fasting blood glucose (FBG), and insulin levels. The homeostasis model assessment of insulin resistance (HOMA-IR) was performed as follows: fasting blood insulin (IU/L) ├Ś FBG (mg/dL) / 405. Body mass index (BMI) was classified according to the Asia-specific criteria into four categories: < 18.5, 18.5ŌĆō22.9, 23.0ŌĆō24.9, and Ōēź 25 kg/m
2. Obesity was defined as having a BMI Ōēź 25 kg/m
2, which is the recommended cutoff value for diagnosing obesity in the Asian population [
21].
Diagnosis of GBP
The abdominal US was performed by experienced radiologists who were blinded to the studyŌĆÖs objectives and clinical data using a 3.5-MHz transducer (LOGIQ 9; General Electric, Madison, WI, USA) to assess GBP only after a minimum fasting period of 10 hours. GBP was diagnosed according to standard radiological criteria when an immobile mucosal lesion protruding into the GB lumen and attached to the GB wall without posterior acoustic shadowing was detected [
22]. The primary endpoint for analysis was the development of GBP categorized by size (Ōēź 5 mm, Ōēź 10 mm, and overall; < 5, 5ŌĆō9, and Ōēź 10 mm).
Statistical analyses
The patientsŌĆÖ baseline characteristics are presented based on HBsAg and anti-HCV status. The primary outcome was GBP development across size categories (Ōēź 5 mm, Ōēź 10 mm, and overall [< 5, 5ŌĆō9, and Ōēź 10 mm]). PatientsŌĆÖ baseline characteristics and GBP prevalence according to HBsAg, HbcAb, and anti-HCV status were compared using the chi-square test and StudentŌĆÖs t-test for categorical and continuous variables, respectively. Data are expressed as mean ┬▒ standard deviation, median (interquartile range), or frequency (%).
Adjusted odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were estimated for the development of GBP in comparison with the reference exposure groups using multivariate logistic regression analysis. The model was first adjusted for age and sex. The multivariable-adjusted model was adjusted for confounders, including age, sex, smoking status, alcohol intake, physical activity (inactive, minimally active, or HEPA), educational level, obesity, fatty liver, metabolic syndrome, aspartate transaminase, alanine aminotransferase, and HOMA-IR. To assess the independent effect of HBcAb positivity on GBP development, we conducted subgroup analyses among HBsAg-negative individuals.
All reported p values were two-tailed, and values of p < 0.05 were considered statistically significant. SPSS version 21 (IBM Corp., Armonk, NY, USA) was used for the statistical analyses.
RESULTS
At baseline, the mean age of the 392,913 participants was 39.3 years (standard deviation, 9.9 years); the proportion of male participants was 53.3%. The prevalence of GBP sized Ōēź 5 mm, Ōēź 10 mm, and overall (< 5, 5ŌĆō9, and Ōēź 10 mm) was 2.9%, 0.1%, and 12.8%, respectively. The overall prevalence of HBsAg, HBcAb, and anti-HCV positivity was 3.2%, 26.7%, and 0.1%, respectively. Among the 380,312 HBsAg-negative participants, the prevalence of HBcAb positivity was 24.3% (
Table 1). Compared with the HBsAg-negative group, the HBsAg-positive group tended to be older and obese; had a higher proportion of men, current smokers, and hypertension; and had fewer individuals with alcohol intake (> 10 g/d for male, > 5 g/d for female) and fatty liver. HBsAg-positive participants also tended to have elevated liver enzyme levels but more favorable lipid levels than HBsAg-negative participants. In the anti-HCV-positive group, the mean age, BMI, proportion of current smokers, hypertension, and diabetes were higher, whereas the proportion of fatty liver was lower than that in the anti-HCV-negative group. Anti-HCV-positive participants also tended to have elevated liver enzyme levels but more favorable low-and high-density lipoprotein cholesterol levels than anti-HCV-negative participants. The proportion of individuals with HEPA of physical activity and met was higher among HBsAg- and anti-HCV-positive participants than among HBsAg- and anti-HCV-negative participants (
Table 2).
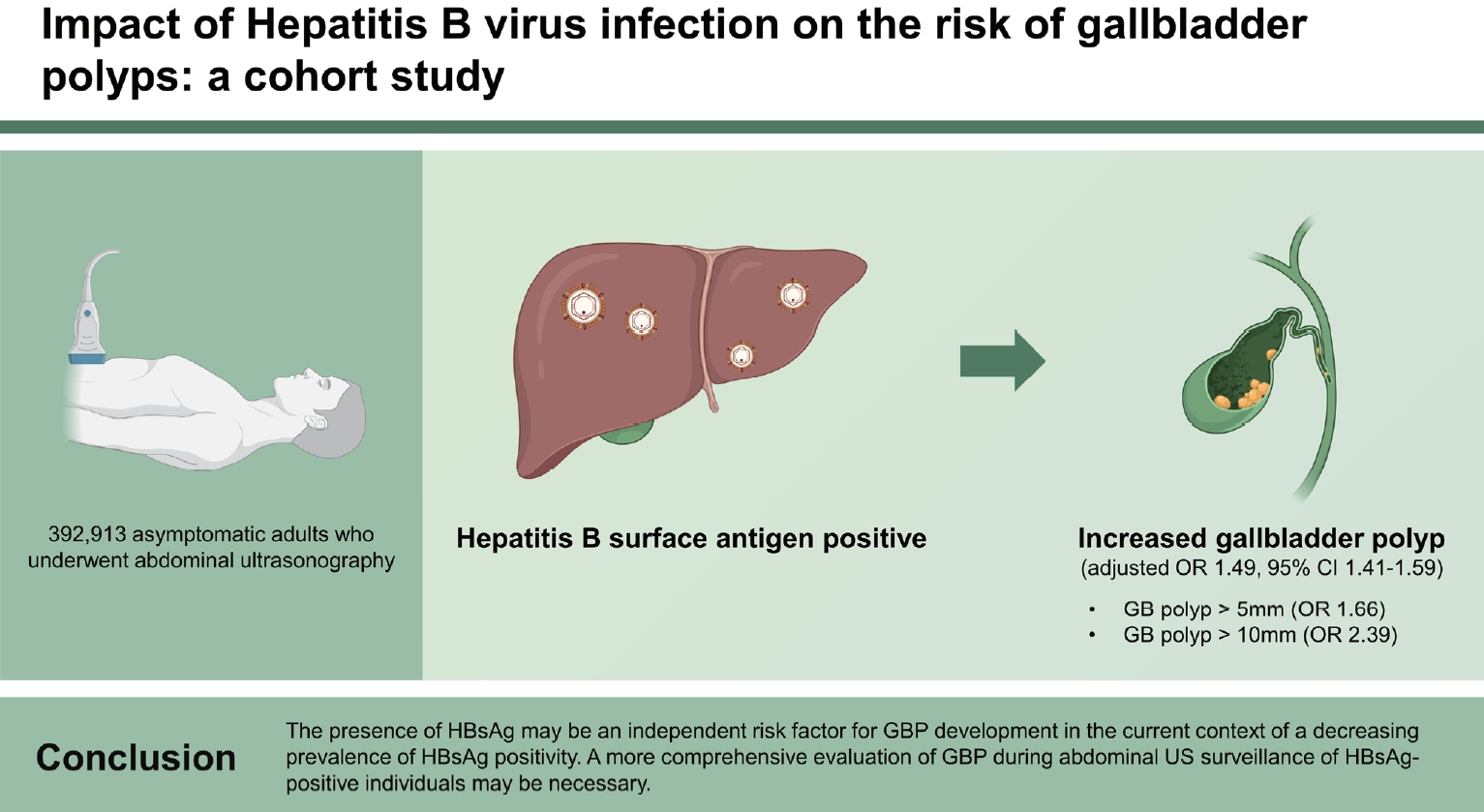
Table 3 shows the association between presence of HBsAg, HBcAb, and anti-HCV, and GBP risk categorized by size (Ōēź 5 mm, Ōēź 10 mm, and overall [< 5, 5ŌĆō9, and Ōēź 10 mm]). After the adjustment for age, sex, smoking status, and other confounders, HBsAg-positive individuals had significantly increased risks of developing GBP of all different sizes, including Ōēź 5 mm (adjusted OR, 1.66; 95% CI, 1.49ŌĆō1.85), Ōēź 10 mm (adjusted OR, 2.39; 95% CI, 1.53ŌĆō3.75), and overall (< 5, 5ŌĆō9, and Ōēź 10 mm; adjusted OR, 1.49; 95% CI, 1.41ŌĆō1.59) (
Table 3,
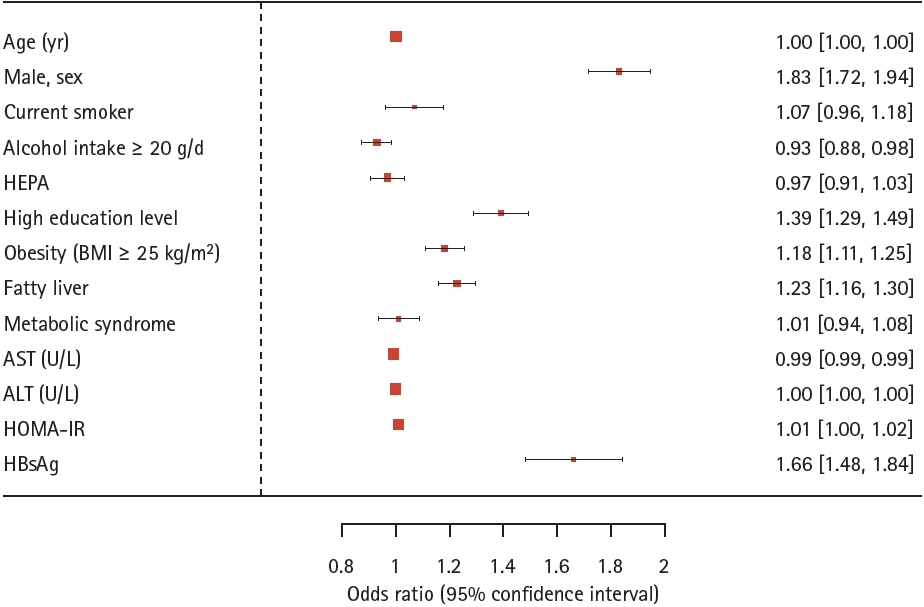
Supplementary Table 1), while anti-HCV individuals did not. Factors such as age, male sex, current smoking status, higher education level, obesity, fatty liver, and elevated HOMA-IR were also found to significantly increase the risk of GBP Ōēź 5 mm, whereas, alcohol consumption and elevated AST level were inversely associated with this risk (
Fig. 2).
HBcAb presence was significantly associated with an increased the risk of GBP with overall size (< 5, 5ŌĆō9, and Ōēź 10 mm; adjusted OR, 1.04; 95% CI, 1.01ŌĆō1.07). However, this association was no longer observed for GBP Ōēź 5 mm or Ōēź 10 mm. In a subgroup analysis of 380,312 HBsAg-negative individuals, the presence of HBcAb was not significantly associated with an increased risk of developing GBP of any size (Ōēź 5 mm, Ōēź 10 mm, and overall [< 5, 5ŌĆō9, and Ōēź 10 mm]) (
Table 4).
DISCUSSION
Here we evaluated GBP risk according to HBsAg, HBcAb, and anti-HCV status in a large-scale cohort of young and middle-aged Korean adults. GBP risk was significantly increased in HBsAg-positive individuals, and the increased risk applied to GBP Ōēź 5 mm, Ōēź 10 mm, and overall (< 5, 5ŌĆō9, and Ōēź 10 mm). In contrast, anti-HCV-positive individuals were not at significantly increased risk of developing GBP. In particular, among HBsAg-negative individuals, the presence of HBcAb did not significantly increase the risk of GBP of any size. Our results indicated that HBsAg is an independent risk factor for GBP development, suggesting that HBV infection may be involved in its pathogenesis.
Several epidemiological studies demonstrated an association between HBV or HCV infection and the development of extrahepatic cancers, including GBC [
23,
24]. A recent nationwide cohort study of Korea reported that individuals with chronic HBV (hazard ratio [HR], 1.55; 95% CI, 1.05ŌĆō2.29) or HCV (HR, 1.46; 95% CI, 1.10ŌĆō1.93) infection are at higher risk of GBC than those without it [
23]. Another prospective cohort study also exhibited an increased risk of GBC or extrahepatic bile duct cancer (HR, 14.89; 95% CI, 10.36ŌĆō21.41) among HBsAg-positive participants [
24]. Both the direct effect of HBV DNA integration into the host genome, which can alter host gene expression and signaling pathways, and the indirect effect of chronic HBV infection, which induces persistent inflammatory hypoxia, angiogenesis, and oxidative stress, are potential mechanisms of HBV-induced GBC [
24-
26]. Accumulating epidemiological evidence demonstrated a link between HBV infection and GBP, indicating that HBV infection increases the risk of GBP, which can progress to GBC [
6,
7,
9,
27]. A study by Yang et al. [
7] of 11,816 healthy screening subjects showed a 2.563- fold increased risk of GBP in HBsAg-positive versus -negative subjects. Another Chinese study of 34,669 healthy participants reported that HBsAg positivity was significantly associated with an increased risk of developing GBP (adjusted OR, 1.113;
p < 0.0005) [
9]. A caseŌĆōcontrol study in Korea, matched for age and sex, also identified HBsAg positivity as an important risk factor for GBP formation (adjusted OR, 3.548; 95% CI, 1.295ŌĆō9.716) [
27]. A recent meta-analysis evaluating risk factors associated with GBP development in an East Asian population demonstrated that HBsAg positivity was a significant risk factor for GBP development [
6]. However, several other studies reported weak or insignificant associations between HBV infection and GBP risk [
14,
15]. A Korean study that evaluated temporal changes in the prevalence and risk factors of GBP over a 10-year period found a significant increase in the prevalence of GBP in 2002ŌĆō2012; the risk factors for GBP also changed over time [
2]. This study demonstrated that HBsAg positivity was a significant risk factor for GBP during the early (2002ŌĆō2004) but not later (2010ŌĆō2012) study period, suggesting that the impact of HBV infection on GBP risk may have decreased over time [
2]. Most previous studies that reported a positive correlation between the presence of HBsAg and GBP risk used data collected many years ago, some as far back as 20 years. It is important to consider potential changes in disease patterns, treatment options, and population characteristics over time when interpreting the relevance and applicability of these findings in the current context. Korea was formerly an HBV-endemic area, but the epidemiology of HBV infection changed with the introduction of HBV vaccinations in 1983, nationwide immunizations in 1995, and advances in antiviral treatment [
28-
30]. The prevalence of HBsAg was 8ŌĆō10% in the 1980s versus 4.6% in the 1990s and has remained around 3.0% since 2010; thus, Korea is now classified as an area of intermediate endemicity [
28,
30]. Consistent with these findings, our large-scale cohort study of recent data reported an HBsAg-positive prevalence of 3.2%. Our study also confirmed a significantly increased risk of GBP in HBsAg-positive individuals, even in the current context of a reduced prevalence of HBsAg positivity, and the increased risk applied to all sizes of GBP including Ōēź 5 mm, Ōēź 10 mm, and overall (< 5, 5ŌĆō9, and Ōēź 10 mm). Furthermore, this study confirmed HBsAg positivity, age, male sex, current smoking, obesity, and fatty liver as independent risk factors for GBP development, thereby corroborating the findings of previous studies [
2,
12,
31].
HBcAb positivity is generally considered indicative of past or persistent HBV infection, and its presence could potentially reflect an unrecognized occult HBV infection, which refers to the presence of HBV DNA without detectable HBsAg [
32]. Hence, the presence of HBcAb can be an important indicator of previous or ongoing HBV infection, even in the absence of detectable HBsAg, and its clinical significance was recently highlighted [
32]. However, there are limited studies on the association between HBcAb and the risk of GBP, with conflicting findings. A Chinese study examining 60,064 asymptomatic screening examinees found a strong positive association between HBcAb (without HBsAg) and GBP (adjusted OR, 2.88; 95% CI, 2.69ŌĆō3.09) [
11], whereas another Chinese study showed no correlation [
14]. The reason for the inconsistent findings on the association between HBcAb+/HBsAg- and GBP is not clear, but the small sample sizes of previous studies may have contributed to this inconsistency. Furthermore, unmeasured confounding factors related to the presence of HBcAb could have affected the development of GBP and contributed to the discrepancy in the results. In this large cohort study, HBcAb positivity in the absence of HBsAg did not increase the risk of developing GBP. Further mechanistic studies are necessary to better understand the relationship between HBV infection and the risk of GBP.
Although studies examining the association between HCV infection and GBP are limited and the impact of anti-HCV positivity on GBP development is unclear, the available evidence suggests that anti-HCV positivity is not a significant risk factor for GBP [
9,
13]. Consistent with previous studies, we found that anti-HCV positivity was not a significant factor in GBP development.
To the best of our knowledge, this is the largest cohort study with the most recent data examining the impact of the presence of HBsAg, HBcAb, and anti-HCV on the development of GBP using high-quality standardized clinical and laboratory indices. Most importantly, our study is the first to assess the risk of GBP by subdividing polyps into different sizes and highlighting the independent contribution of HBsAg positivity to GBP development.
However, the current study has several limitations that require consideration. First, this was a hospital-based rather than a population-based study, and our data consisted mostly of young, middle-aged, and relatively healthy Koreans. Hence, our findings may not be generalizable to older groups or other ethnicities. However, previous epidemiological studies have reported that the highest incidence of GBP occurs in individuals aged 30ŌĆō50 years [
1,
33]. This may be attributed to the fact that most individuals who have undergone cholecystectomy are in their 50s, 60s, or older [
2]. Therefore, our study cohort of predominantly young and middle-aged individuals may be better suited to identify risk factors for GBP. Second, the histological classification of GBP was not performed, which could have improved the accuracy and reliability of identifying its predictors. Differences may exist in the formation and pathological processes of cholesterol and non-cholesterol GBP [
34]. Nonetheless, this study assessed the risk of GBP with a size of Ōēź 5 mm and Ōēź 10 mm, in addition to overall size since data on GBP Ōēź 5 mm may have greater clinical significance. This is because a GBP < 5 mm can be challenging to differentiate from gallstones or is unlikely to be a neoplastic polyp in most cases. Third, this study did not assess inter-observer variations in the sonographic diagnosis of GBP. However, all sonographic examinations were conducted by skilled board-certified radiologists using the same classification system with a well-defined definition of GBP. Fourth, information about specific antiviral drugs used to treat hepatitis that could affect GBP development was not included in this study. Finally, the cross-sectional design of this study does not allow the establishment of causal or temporal relationships. The presence of HBsAg, HBcAb, and the anti-HCV status may change over time; however, most patients were evaluated only once (during the initial visit). Therefore, the impact of dynamic changes in hepatitis virus status on GBP risk could not be assessed here. Nevertheless, most patients with chronic viral hepatitis requiring treatment are not typically included in asymptomatic health checkups, and the large sample size in this study may mitigate the potential influence of disease fluctuation bias. Despite these limitations, our findings provide valuable insight into the distinct roles of HBsAg, HBcAb, and anti-HCV in GBP risk.
In conclusion, HBsAg positivity may be an independent risk factor for GBP, even in the current context of the declining prevalence of HBsAg positivity. Our results suggest that HBV infection is involved in the pathogenesis of GBP. Given the association between HBsAg and GBP, it may be necessary to perform more thorough screening for GBP during surveillance abdominal US among HBsAg-positive patients. Further large-scale prospective studies are needed to elucidate whether HBV treatment contributes to GBP regression or reduces its development and consequences.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement table 1
Supplement table 1 Print
Print


