INTRODUCTION
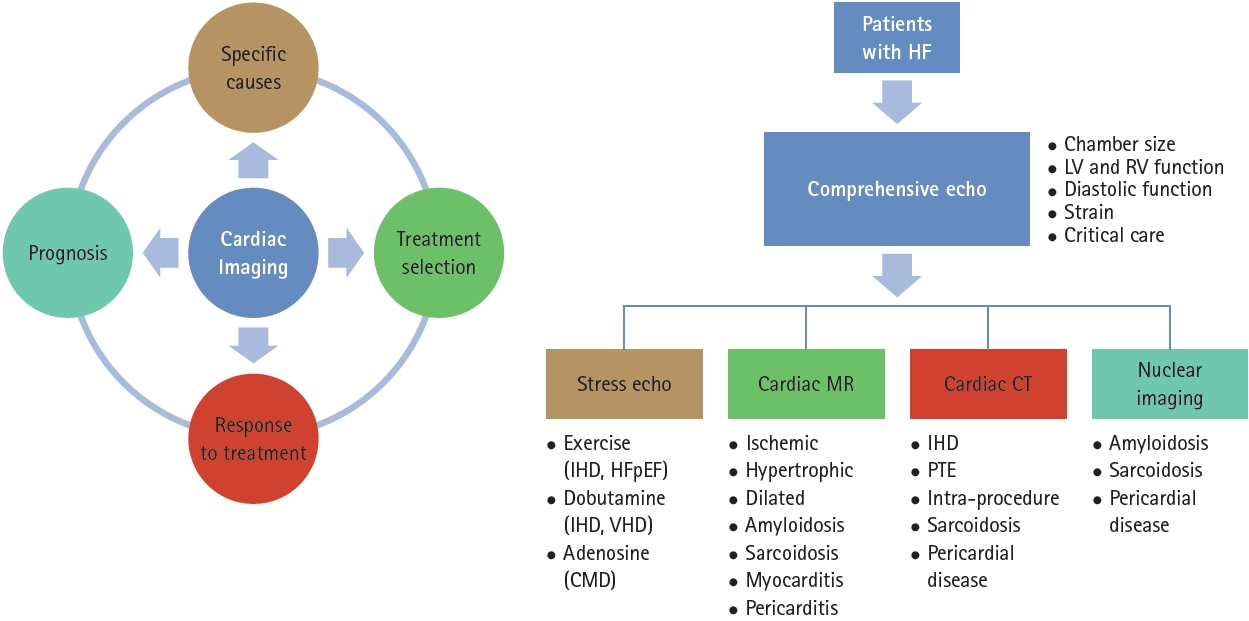
Cardiac imaging is an essential modality for identifying and treating patients with heart failure (HF). Because HF is a complex clinical syndrome that can result from a wide range of cardiac conditions [1], and different cardiac imaging techniques have unique but complementary roles, a multimodal imaging approach is crucial for identifying the underlying causes of HF and enabling personalized treatment and prognostication. Significant advances have been made in cardiac imaging to identify the specific causes of HF and guide the management of critically ill patients. In this review, we comprehensively addressed the role of cardiac imaging in the management of HF.
ECHOCARDIOGRAPHY
Routine evaluation
Echocardiography is the first-line bedside tool for diagnosing HF [2]. It helps determine the left ventricular ejection fraction (LVEF), which is used to divide HF into the following three phenotypes: HF with preserved ejection fraction (HFpEF, LVEF ≥ 50%); HF with mildly reduced ejection fraction (HFmrEF, 40% ≤ LVEF < 50%); and HF with reduced ejection fraction (HFrEF, LVEF < 40%) [3]. Because treatment approaches are different and disease-modifying therapy can be initiated based on the different phenotypes of HF, accurate estimation of LVEF remains the cornerstone of HF diagnosis. Two-dimensional LVEF is typically calculated using the modified biplane Simpson method, while three-dimensional echocardiographic assessment is more likely to provide volumes comparable with those provided by cardiac magnetic resonance (CMR).
The etiology of HF can also be determined using echocardiography. Substantial valvular dysfunction and regional wall motion abnormalities, which, in the appropriate clinical context, may indicate an ischemic etiology of HF, can be identified using echocardiography. An increased wall thickness caused by hypertrophy in response to pressure load, cardiomyopathy, or infiltrative disorders can also be quantified by echocardiography. Transesophageal echocardiography is useful when thrombosis, prosthetic valve dysfunction, endocarditis, or congenital heart disease are suspected.
Hemodynamic evaluation using Doppler imaging
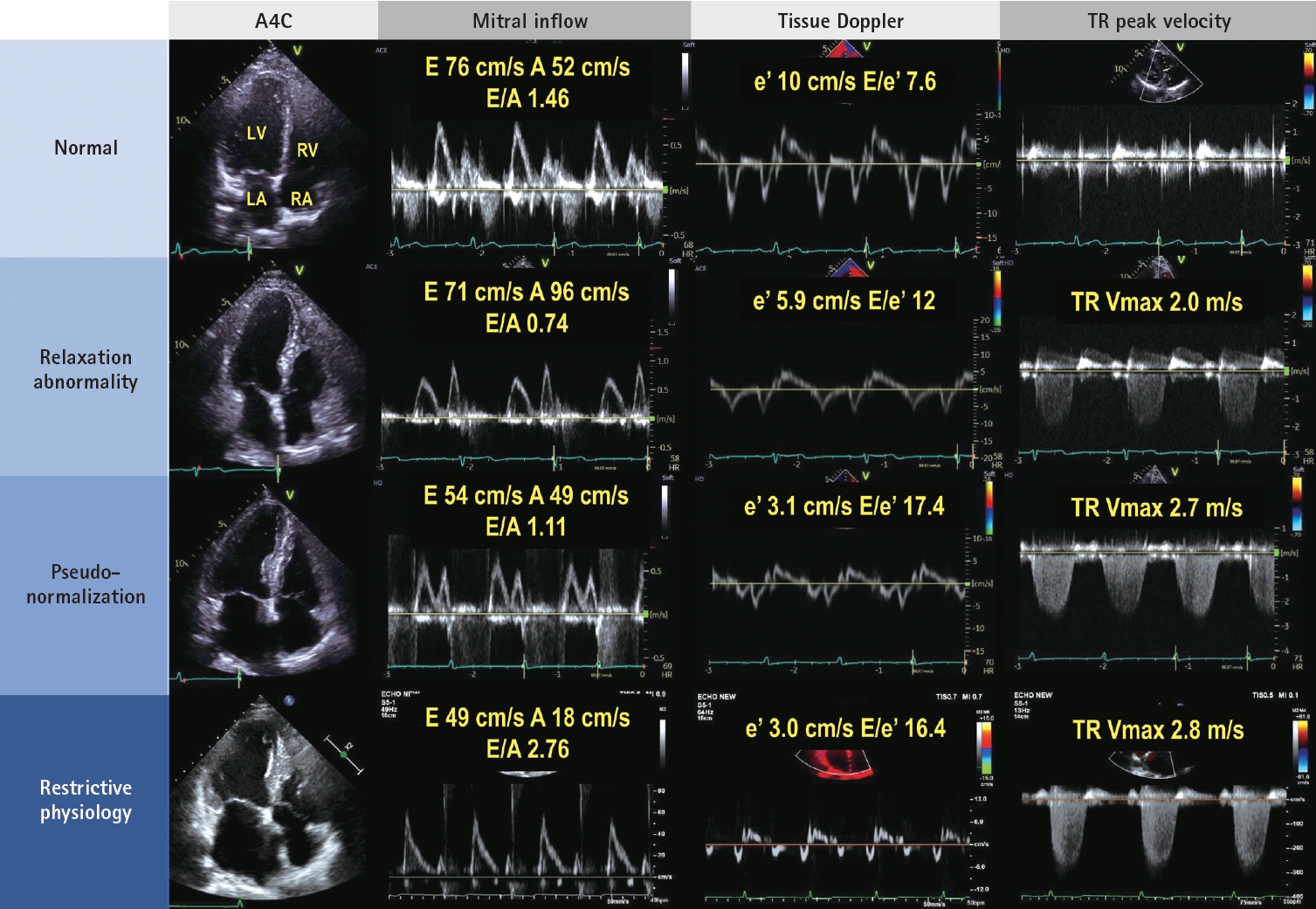
Hemodynamic evaluation using Doppler imaging is essential in comprehending the overall condition of patients with HF and managing them appropriately. Although LVEF is preserved, dyspnea can be attributed to a heart problem known as HFpEF. Recent guidelines have used several scores to diagnose HFpEF, most of which include echocardiographic parameters related to diastolic function and morphological changes. Regarding diastolic function or functional testing, mitral annular e’ velocity (septal e’ < 7 cm/s or lateral e’ < 10 cm/s), average E/e’ (major ≥ 15, minor ≥ 9), and peak tricuspid regurgitation (TR) velocity > 2.8 m/s are used. For morphological testing, an increased left atrial (LA) maximal volume index (major: > 34 mL/m2 in sinus, > 40 mL/m2 in atrial fibrillation, minor: ≥ 29 mL/m 2 in sinus, ≥ 34 mL/m 2 in atrial fibrillation) and the presence of cardiac hypertrophy are utilized [4,5].
Understanding the relationship between diastolic parameters is essential for further understanding the hemodynamic status of patients with HF. E’ is utilized as an index of left ventricular (LV) relaxation. Slow relaxation leads to a reduction in the early diastolic transmitral pressure gradient, resulting in a decreased mitral E velocity, a low E/A velocity ratio, and prolonged E-deceleration time [6]. As LV diastolic dysfunction worsens, LA pressure increases to maintain the transmitral pressure gradient, leading to an elevation in the LV filling pressure (Fig. 1). LV filling pressure is closely associated with the symptoms of HFpEF, making it crucial to estimate LV filling pressure while considering the clinical signs and additional laboratory tests. The relationship between diastolic function parameters and LV filling pressure is modified in various diseases and should be interpreted carefully, particularly in cases of hypertrophic cardiomyopathy (HCM), valvular heart disease (including mitral stenosis and aortic regurgitation), mitral annulus calcification, heart transplantation, and atrial fibrillation [7-9].
Although patients may have normal resting LV filling pressure, they can experience symptoms during exercise. In such cases, an echocardiographic stress test can be used to confirm HFpEF and take further steps in managing them. If the average E/e’ is > 15 and TR velocity is > 3.4 m/s during exercise, HFpEF can be confirmed [4].
Strain
Strain is defined as the change in the length of a myocardial segment relative to its resting length [10]. Global longitudinal strain (GLS) may be more sensitive than LVEF in detecting early myocardial disease in patients with cardiovascular risk factors [11,12], and in predicting risk beyond LVEF in patients with HFrEF [13]. Reported normal values of GLS varied from -15.9 to -22.1% (mean, -19.7%) in a meta-analysis [14], therefore, absolute values of GLS < 16–18% are consistent with LV systolic dysfunction [6]. Additionally, longitudinal deformation patterns can assist in the differential diagnosis of patients with LV hypertrophy (Fig. 2). Furthermore, LA reservoir strain can detect LV diastolic alterations and elevated LV filling pressure, even when the LA maximal volume index is normal.
Subclinical myocardial ischemia can occur before a reduction in LVEF in patients receiving cardiotoxic chemotherapy [15], particularly affecting the endocardial layer of the myocardium [16]. According to recent cardio-oncology guidelines, it is recommended to assess the baseline LVEF and GLS through echocardiography in all patients before initiating cardiotoxic cancer treatment. This was performed to evaluate the risk of cardiovascular toxicity associated with cancer therapy and to monitor any significant changes in heart function during treatment [17]. Cancer therapy-related cardiac dysfunction (CTRCD) is defined as a relative GLS decrease of 15% or an absolute reduction of LVEF > 10% to < 55% compared with the baseline. A recent randomized controlled study compared GLS-guided and LVEF-guided cardio-protection in survivors of potentially cardiotoxic chemotherapy. The study demonstrated the potential benefits of the GLS-guided approach in preventing CTRCD at the 1-year follow-up but found no significant difference between the two strategies at the 3-year follow-up [18,19]. These findings challenge the routine use of the GLS-based strategy in patients undergoing cardiotoxic chemotherapy and suggest a more selective approach for imaging surveillance.
Assessment of the right ventricle
Although the focus of clinical assessments has often been on the LV, it is crucial to equally focus on the right ventricle (RV) for the management of HF. The RV is more vulnerable to pressure and volume overload; therefore, LV dysfunction is often accompanied by RV dysfunction. In clinically unstable patients, identifying RV dysfunction and observing a D-shaped LV using echocardiography are vital. Efforts should be made to identify and treat the underlying causes of RV failure, such as acute pulmonary embolism, acute respiratory distress syndrome, and acute decompensation of chronic pulmonary hypertension. RV failure can also result from a primary reduction in myocardial contractility caused by ischemia, cardiomyopathy, or arrhythmia [20]. On echocardiography, RV function can be visually assessed, while fractional area change, tricuspid annular plane systolic excursion, and tissue Doppler S’ provide more quantitative assessments [21]. Additionally, assessing the peak velocity of TR is necessary to identify accompanying pulmonary hypertension.
Myocardial contrast echocardiography
The myocardial signal intensity emanating from the contrast agent indicates the concentration of microbubbles in the myocardium. The signal intensity reflects the relative capillary blood volume when the myocardium is fully saturated with microbubbles. After a brief burst of high-power imaging that clears the microbubbles from the myocardium, their replenishment can be observed. In a normal myocardium with a normal coronary artery, the contrast appears within 5 s during replenishment at rest and within 1–2 s during stress, when the myocardial blood flow increases. The delayed appearance of contrast with reduced intensity in the subendocardium is a sign of flow-limiting coronary artery disease (CAD) caused by reduced blood flow velocity and decreased capillary blood volume [22].
Intracoronary myocardial contrast echocardiography can also assist in selecting candidates for alcohol septal ablation, a treatment option for HCM. This helps physicians confirm whether the selected branch to occlude supplies the target area for treating systolic anterior motion without opacifying other remote areas [23].
Stress echocardiography
Exercise stress echocardiography
Exercise stress echocardiography aids in identifying inducible ischemia in individuals with suspected ischemic heart disease, evaluating the myocardial reserve in patients with reduced LV and RV systolic function, and observing symptom development in patients with valvular heart disease [24,25]. Additionally, diastolic stress echocardiography can detect elevated filling pressure during exercise, assisting in the diagnosis of HFpEF [4,26], as mentioned previously.
Dobutamine stress echocardiography
Dobutamine stress echocardiography (DSE) is a valuable tool for detecting myocardial reserve and inducible ischemia in patients with CAD. Assessment of myocardial viability and identification of hibernating myocardium using DSE can guide decisions regarding revascularization in patients with an ischemic etiology.
DSE is commonly used to evaluate valvular heart disease, particularly aortic stenosis (AS). Severe AS can be diagnosed when the peak velocity is ≥ 4 m/s, mean pressure gradient is ≥ 40 mmHg, and the aortic valve area is < 1.0 cm2. However, these parameters are not always consistent. Additionally, in patients with decreased LVEF (< 50%), the aortic valve area can be < 1.0 cm 2 with low flow and gradient. This can also occur in patients with preserved LVEF but decreased stroke volume, known as a paradoxical low-flow, low-gradient. DSE is highly valuable in distinguishing between true severe AS and moderate AS with a small opening of the aortic valve due to a low stroke volume. It can help identify patients requiring intervention for AS and determine the timing of the intervention [27].
Adenosine stress echocardiography
Coronary microvascular dysfunction (CMD) is common in patients with HFpEF, and those with CMD are at a higher risk of developing HF during follow-up [28,29]. By adenosine stress echocardiography, we can assess the coronary blood flow from the distal left anterior descending artery using Doppler and identify the presence of CMD [30].
Critically ill patients
Patients with unstable vital signs often exhibit cardiac dysfunction, which can stem from chronic underlying conditions or the acute disease itself [31]. Comprehensive initial assessment and ongoing reevaluation of the treatment response of a patient are critical [32]. The evaluation begins with a detailed clinical examination and assessment of the available physiologic variables [33]. Bedside echocardiography offers a more comprehensive evaluation of hemodynamic problems, and is noninvasive, portable, and safe; provides immediate results; and allows for repeated measurements. However, adequate scanning can be challenging due to factors such as mechanical ventilation, drains, wound dressings, suboptimal positioning, and dynamic changes in patient status. Despite these challenges, echocardiography can provide crucial information for critically ill patients, and the results should be applied promptly in the decision-making process.
Shock status
Bedside echocardiography allows for the rapid assessment of volume status, LV and RV function, substantial valvular dysfunction, and pericardial effusion, even with only a glance at the parasternal long-axis view. Obliteration of the LV cavity with hyperdynamic motion may indicate hypovolemia, whereas a D-shaped LV in an appropriate clinical context may suggest pulmonary thromboembolism in the absence of severe pulmonary disease [34].
During septic shock, the inflammatory response leads to a decrease in systemic vascular resistance. To maintain adequate tissue perfusion, the heart initially compensates by pumping forcefully. However, cardiac dysfunction, also known as stress-induced cardiomyopathy, can occur as shock progresses. This condition is associated with different anatomical patterns, including apical ballooning (Takotsubo), basal (inverted Takotsubo), midventricular, and biventricular patterns. In the case of an apical ballooning pattern, LV outflow tract obstruction and mitral regurgitation may occur, accompanied by hyperdynamic motion of the basal walls. Therefore, close monitoring is necessary to observe the development of LV outflow tract obstruction, mitral regurgitation, and thrombus formation at the apex [35].
Extracorporeal membrane oxygenation
Echocardiography provides valuable information both before and during extracorporeal membrane oxygenation (ECMO). A thorough echocardiographic evaluation is essential when assessing the condition of the patient before initiating ECMO support. The evaluation focuses on chamber size and morphology, LV and RV function, and significant valvular dysfunction, particularly aortic regurgitation, pericardial effusion, and vascular pathology, such as aortic dissection. In extreme situations, such as extracorporeal cardiopulmonary resuscitation for refractory cardiac arrest, echocardiography can rapidly provide anatomical information about the heart.
Serial echocardiography during ECMO primarily focuses on monitoring cardiac chamber size to ensure proper ventricular emptying. In peripheral venoarterial ECMO, retrograde aortic blood flow competes with the stroke volume ejected from the LV, potentially leading to LV distention and thrombus formation when the aortic valve is closed. Therefore, it is crucial to assess the aortic valve opening. In cases of severe LV and LA dilatation with associated pulmonary edema, LA venting for LV decompression can be performed, resulting in rapid resolution and improvement [36].
Response to treatment
The extent and pattern of remodeling in patients with HF can serve as predictors of clinical deterioration. While LVEF is the most commonly measured variable during follow-up, both the LV end-diastolic volume index and GLS are valuable markers [37,38]. High pulmonary artery pressure is associated with a significant increase in mortality, hospitalization, and the likelihood of cardiac transplantation [39,40]. In one study, LA strain was found to enhance the detection of diastolic dysfunction and was linked to worse functional status and an increase in HF hospitalizations [41].
In treating patients with HF using guideline-directed medical therapy (GDMT), it is important to assess LV systolic function to determine the potential need for a cardiac device, such as an implantable cardioverter-defibrillator or cardiac resynchronization therapy. The consideration for cardiac device implantation arises in patients with persistent symptoms and a reduced LVEF < 35% based on specific indications, after at least 3 months of GDMT, who are expected to survive for at least 1 year with good functional status [42].
CARDIAC MAGNETIC RESONANCE IMAGING
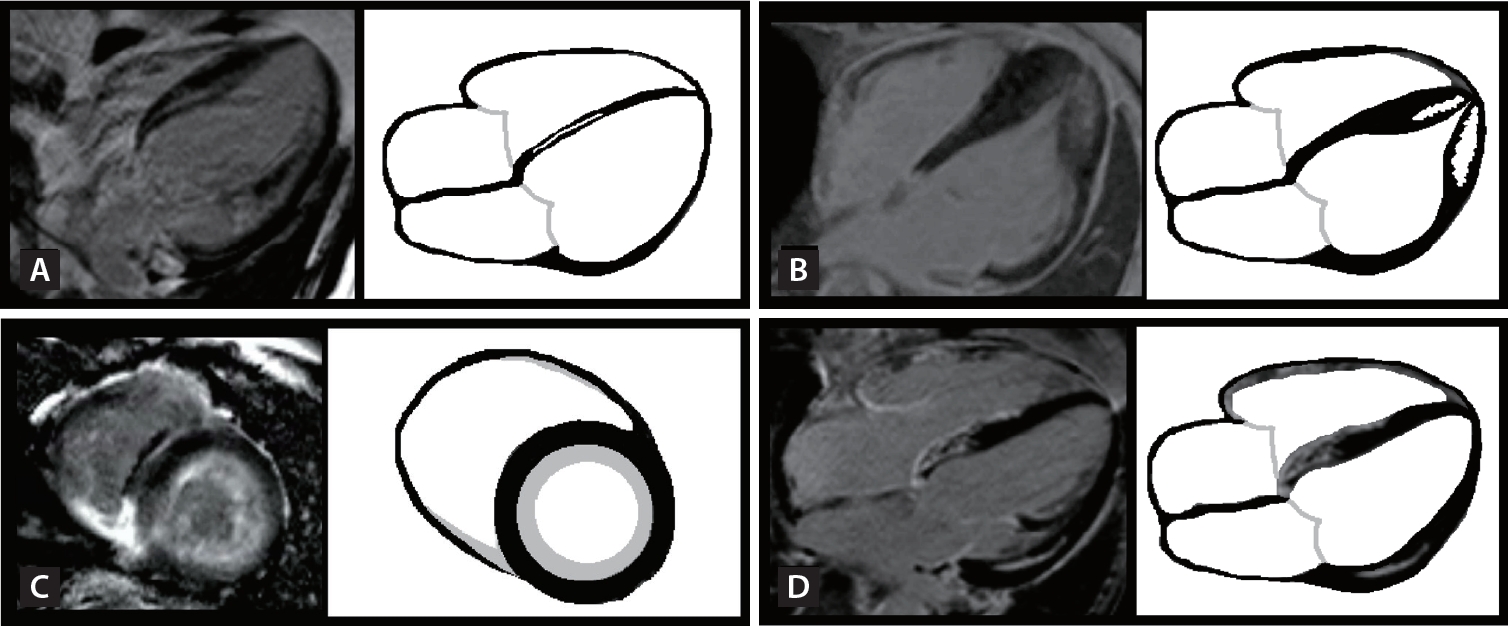
CMR is considered the gold standard for measuring ventricular volume and LVEF. It is particularly suitable for assessing the RV, which can be challenging to visualize using echocardiography. Additionally, CMR is the only imaging technique that provides detailed tissue characterization of the myocardium and allows for the assessment of myocardial fibrosis [43,44]. Various tissue characterization techniques have been employed in CMR, including the inversion recovery images acquired either early or late after contrast administration, T1 mapping for diffuse fibrosis assessment, extracellular volume (ECV) measurement, T2-weighted images for edema evaluation, and T2* for iron concentration measurement. Late gadolinium enhancement (LGE) refers to a bright signal observed due to delayed washout of gadolinium in areas of myocardial scarring or replacement fibrosis compared to the normal myocardium. The presence, distribution, and extent of LGE provide important diagnostic information for HF. It aids in identifying silent myocardial infarction and specific cardiomyopathies, such as amyloidosis, HCM, sarcoidosis, cardiac hemochromatosis, and constrictive pericarditis. The different patterns of LGE and parametric mapping corresponding to different etiologies of HF are summarized in Figure 3.
Ischemic cardiomyopathy
Infarct-related LGE is typically found in the subendocardial area and becomes more transmural as infarct severity worsens. They are typically confined to one or more coronary territories [45]. The extent of scar transmurality is crucial in differentiating between viable and nonviable myocardium. If the transmural extent is < 50%, it is considered viable, whereas if it exceeds 50%, it is considered non-viable [46]. The extent of LGE is an important predictor of patient prognosis. In individuals who have experienced a heart attack, the number of segments with a transmural infarct can indicate the extent of LV remodeling and the likelihood of functional recovery following treatment [47,48]. Furthermore, the presence of microvascular obstruction indicates a higher risk of poor functional recovery and future adverse events [49].
Hypertrophic cardiomyopathy
The patterns of enhancement in HCM are diverse and can be categorized into two main types: focal mid-wall LGE at the RV insertion points, indicating interstitial fibrosis and/or myocyte disarray; and intramural LGE within hypertrophied segments, indicating replacement fibrosis [23]. In patients with HCM who are not otherwise considered to be at high risk of sudden cardiac death, CMR imaging is valuable for assessing the maximum LV wall thickness, LVEF, presence of LV apical aneurysm, and extent of myocardial fibrosis through LGE [50]. Extensive LGE, constituting ≥ 15% of the LV mass, indicates a high risk of sudden cardiac death in HCM.
Dilated cardiomyopathy
In dilated cardiomyopathy, the myocardial wall becomes thinner, and there is a considerable decrease in LVEF. Dilated cardiomyopathy typically exhibits diffuse mid-wall or subepicardial LGE, often localized to the inferoseptal wall [51]. In patients with nonischemic cardiomyopathy, myocardial scarring strongly correlates with an increased risk of all-cause and cardiac mortality [52].
Cardiac amyloidosis
In typical cases of cardiac amyloidosis, CMR reveals a global subendocardial distribution or patchy subendocardial or transmural LGE commonly observed in the LV and both atria simultaneously. Elevated ECV is typically a result of excessive collagen deposition in fibrotic regions, and significant expansion of the extracellular space is characteristic of cardiac amyloidosis [53,54]. Native T1 mapping and ECV quantification also demonstrate prognostic value in cardiac amyloidosis [55].
Cardiac sarcoidosis
In cardiac sarcoidosis, the basal portion of the ventricular septum is the most commonly affected site. Cine magnetic resonance imaging (MRI) can be used to detect typical morphological abnormalities, including basal thinning of the ventricular septum, localized myocardial thickening, ventricular aneurysms, LV regional wall motion inconsistent with coronary blood flow, diffuse myocardial thinning, and LV dilatation (dilated cardiomyopathy-like pattern). LGE is frequently observed in the septum near the base of the heart, in the lateral wall, and predominantly in the epicardium. T2-weighted and early enhancement images can reveal changes attributed to myocardial edema. Extracardiac findings, such as hilar lymphadenopathy and lung lesions, may also be observed [56].
Myocarditis
CMR is appropriate for diagnosing myocarditis in clinically stable patients. CMR scans can reveal a combination of myocardial edema and inflammatory myocardial injury. The diagnostic criteria involve at least one T2-based measure (such as a global or regional increase in myocardial T2 relaxation time or increased signal intensity in T2-weighted CMR images) along with at least one T1-based criterion (such as increased myocardial T1, ECV, or LGE) [57]. A typical LGE pattern in myocarditis is mid-wall/subepicardial LGE in the lateral, inferolateral, or inferior wall.
Pericarditis
CMR plays a crucial role in the diagnosis of atypical manifestations of constrictive pericarditis, such as minimally thickened pericardium, effusive-constrictive pericarditis, and transient constrictive pericarditis. When the pericardium shows enhancement on LGE imaging, physicians can consider administering anti-inflammatory therapy to counteract the active inflammation, potentially reducing the need for surgery [58]. Additionally, CMR can identify concurrent myocardial involvement in the pericardial disease or pericardial injury following myocardial infarction.
CARDIAC COMPUTED TOMOGRAPHY
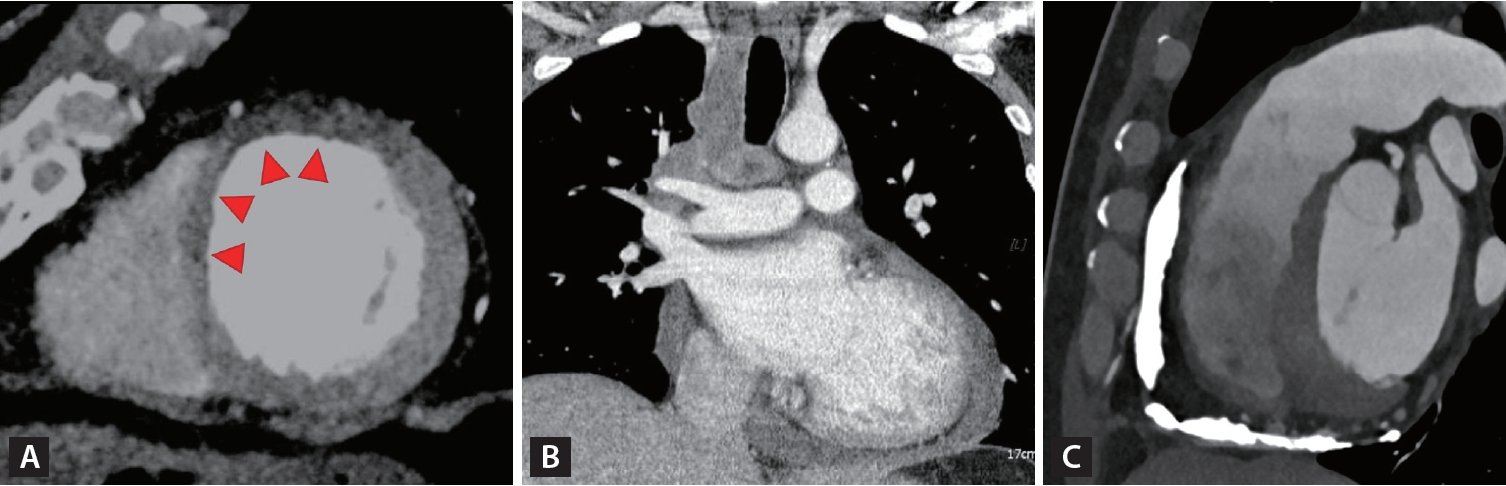
The use of coronary computed tomography angiography (CTA) and noninvasive functional imaging techniques is valuable for identifying the ischemic origin of HF. These techniques are recommended as the initial tests for diagnosing CAD according to the recent guidelines for chronic coronary syndromes [59]. Coronary CTA is the preferred test for patients with a low clinical likelihood of CAD and no prior CAD diagnosis. It can accurately rule out significant CAD. However, coronary CTA is not recommended for cases with extensive coronary calcification, irregular heart rate, significant obesity, or inability to cooperate with breath-hold commands. In cases where there’s a high clinical likelihood of CAD, symptoms unresponsive to medical therapy, typical angina at a low exercise level, and an initial evaluation indicating high event risk, invasive coronary angiography is directly recommended. Multidetector computed tomography (CT) can accurately measure infarct size and provide results comparable to CMR measurements, making it a valuable alternative for patients with non-MRI conditional pacemakers or defibrillators [60,61].
In the recent era of prevalent valvular procedures, CT has become essential as evolving interventional techniques such as transcatheter aortic valve implantation. During the planning phase, CT is used to assess annulus size, height from the annulus to the coronary ostium, puncture site, and presence of atherosclerotic plaques along the aorta. These evaluations are crucial for preventing periprocedural complications and achieving optimal procedural and clinical outcomes.
With a larger field of view, CT scans are valuable for diagnosing specific cardiomyopathies. Chest CT can reveal hilar or mediastinal lymphadenopathy, which is present in approximately 50 to 60% of patients with cardiac sarcoidosis [56]. CT scans play a role in the management of pericardial diseases [58]. In acute pericarditis, a CT scan can detect a thickened pericardium (> 3–4 mm), which is enhanced after contrast administration. It can also identify direct tumor invasion or metastatic spread to the pericardium as well as hidden malignancy in cases of unexplained recurrent pericardial effusion. Because CT is the most accurate technique for imaging calcified tissues, it is essential to perform preoperative workups for constrictive pericarditis, particularly to assess the extent of calcifications. The various roles of CT in the management of HF are shown in Figure 4.
NUCLEAR IMAGING
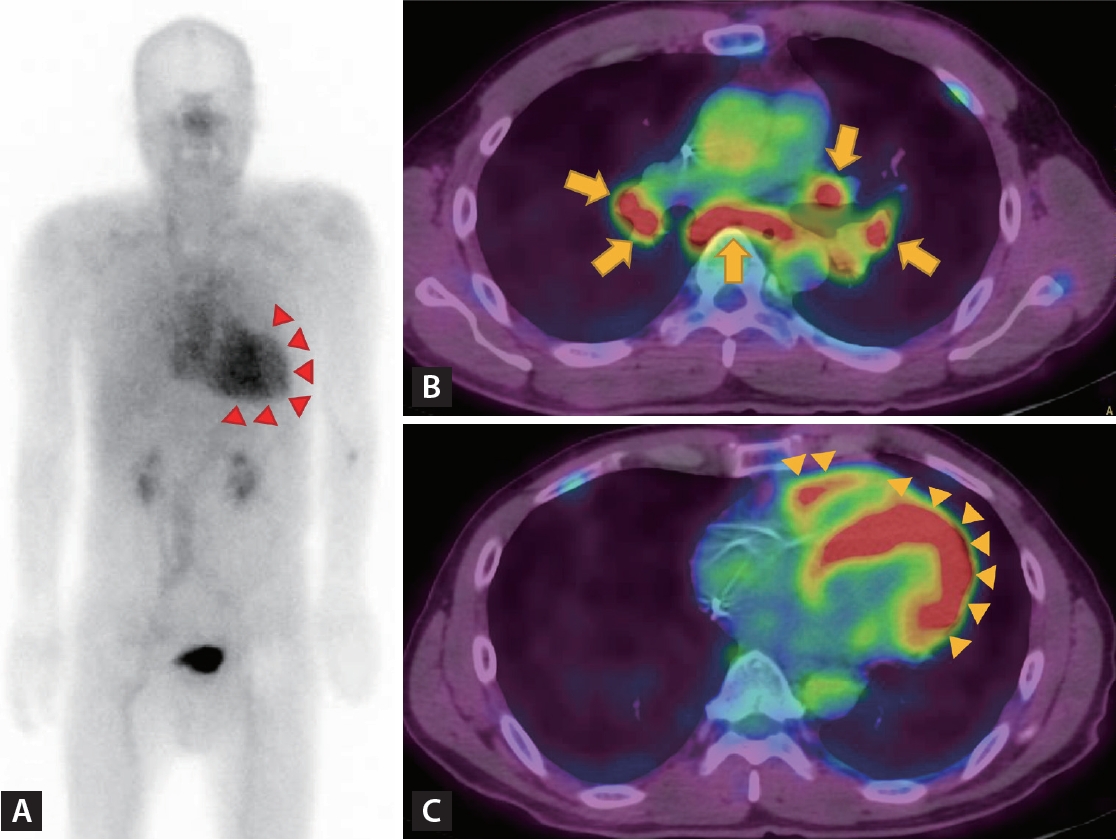
Nuclear imaging plays multiple roles in identifying the etiologies and managing HF, as illustrated in Figure 5.
Ischemic heart disease
Myocardial perfusion imaging with either single-photon emission CT or positron emission tomography (PET) is an established modality for assessing the presence and severity of CAD, aiding in the identification of ischemic origin of HF. This involves visually assessing radiotracer uptake in different segments of the heart during both rest and stress conditions. When there are perfusion defects during stress but not at rest, it indicates reversible ischemia, while fixed defects indicate a myocardial infarction [62]. Moreover, a combination of perfusion and metabolic imaging can detect dysfunctional but potentially recoverable ischemic heart tissue, which may regain contractile function after revascularization [63]. The presence of preserved or increased uptake of 18F- Fluorodeoxyglucose (FDG) alongside reduced resting myocardial perfusion, referred to as flow-metabolism mismatch, strongly suggests the possibility of regional functional recovery after revascularization [64]. Additionally, quantifying myocardial blood flow offers a better understanding of the extent and severity of ischemia in cases of multi-vessel disease. Reduced myocardial blood flow can also identify CMD in the absence of significant epicardial CAD.
Cardiac amyloidosis
Nuclear techniques are also valuable for diagnosing cardiac amyloidosis. Scintigraphy with bone- avid radiotracers, 99m Tc-pyrophosphate (PYP), 99m Tc-3,3-diphosphono-1,2-propanodiacarboxylic acid (DPD), and 99m Tc-hydroxymethylene diphosphonate (HMDP), which bind to microcalcification associated with transthyretin fibrils, is used to identify transthyretin amyloid cardiomyopathy (ATTR-CM) [63]. Given the relatively common occurrence of transthyretin amyloid cardiomyopathy (ATTR-CM) and cardiac involvement in amyloid light-chain amyloidosis, patients with HFpEF should be screened for cardiac amyloidosis under certain circumstances. These circumstances include increased LV wall thickness with no other explanation, a granular sparkling texture of the myocardium, bi-atrial enlargement and pericardial effusion, reduced LV GLS with apical sparing, and bilateral carpal tunnel syndrome [65-67]. Scans are analyzed semi-quantitatively or by visual grading, myocardial uptake of the tracer equal or higher than in the ribs (Grades 2 and 3) indicating the presence of ATTR [63].
Cardiac sarcoidosis
18F-FDG-PET is a valuable tool for diagnosing and treating cardiac sarcoidosis. Imaging modalities help to determine the severity and prognosis of the disease, stage the disease, assess the effectiveness of immunosuppressive treatment, and detect disease flare-ups. Focal myocardial uptake of 18F-FDG indicates active inflammation caused by the infiltration of inflammatory cells [68]. To accurately detect cardiac sarcoidosis lesions, it is important to minimize physiological 18F-FDG uptake by the myocardium, which requires detailed preparation. Patients must fast for 18 h before the examination, consume a high-fat (> 35 g) and low-carbohydrate (< 5 g) diet, and receive a heparin injection immediately before the examination, to increase free fatty acid levels in the blood [56]. Given that increased 18F-FDG uptake can be seen in various other inflammatory conditions and hibernating myocardium, it is advisable to interpret this finding in conjunction with clinical probability, chest CT, and CMR findings when establishing a diagnosis of cardiac sarcoidosis.
Pericardial disease
PET imaging can be used to detect active inflammation in pericardial diseases. In patients with solid cancers and lymphoma, the uptake of 18F-FDG in the pericardium indicates malignant involvement of the pericardium, which aids in the diagnosis, staging, and assessment of therapeutic responses. Uptake is typically intense and often associated with a focal soft tissue mass. PET/CT is also valuable in determining the nature of inflammatory pericarditis, with tuberculous pericarditis showing higher FDG uptake than idiopathic pericarditis.
CONCLUSIONS
Cardiac imaging is crucial for the diagnosis, assessment of etiology, treatment planning, and prognostication of patients with HF. Echocardiography is a primary modality for assessing LV volume and function, quantifying valvular disease, and evaluating hemodynamic status in critical settings. CMR is highly useful for characterizing tissues, including scars, and for diagnosing specific cardiomyopathies. By appropriately utilizing cardiac imaging, healthcare professionals can gain a better understanding of the conditions of their patients, make prompt informed decisions, and provide better overall care to patients with HF, resulting in improved outcomes (Fig. 6).




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





