 |
 |
| Korean J Intern Med > Volume 38(4); 2023 > Article |
|
See editorial "Role of potassium-competitive acid blockers in eradication of Helicobacter pylori infection" on page 451.
Abstract
Background/Aims
Tegoprazan, a novel potassium-competitive acid blocker, has shown rapid action and gastric acid inhibition. In this study, we evaluated the efficacy of a tegoprazan-based, nonbismuth-containing quadruple (concomitant) therapy for the primary eradication of Helicobacter pylori.
Methods
We conducted a prospective, single-arm, single-center, primitive study to verify the efficacy of a 10-day tegoprazan-based (50-mg dose) concomitant therapy, including amoxicillin (1,000-mg dose), clarithromycin (CLA; 500-mg dose), and metronidazole (MET; 500-mg dose) twice daily as a first-line treatment for H. pylori eradication.
Results
We tested consecutive cultures for antibiotic susceptibility and minimum inhibitory concentrations. We enrolled 84 participants; 79 (94.0%) completed first-line therapy. The overall intention-to-treat and per-protocol eradication rates were 90.5% (95% confidence interval [CI], 82.1ŌĆō95.8) and 96.2% (95% CI, 83.4ŌĆō97.6), respectively. Of the 73 participants evaluated for antibiotic resistance, 19 (26.0%), 32 (42.5%), and 8 (11.0%) exhibited CLA, MET, and CLA and MET dual resistance, respectively. Of these, 39 participants (66.1%) exhibited successful eradication after the therapeutic regimen despite antibiotic resistance.
Helicobacter pylori is the pathogen primarily responsible for benign peptic ulcers, atrophic gastritis, intestinal meta-plasia, and gastric cancer [1]. The effectiveness of the most commonly recommended regimens, particularly the triple regimen comprising the antibiotics clarithromycin (CLA) or metronidazole (MET) and amoxicillin (AMX) with a proton pump inhibitor (PPI), has decreased owing to increased resistance to CLA and MET [2ŌĆō4]. To enhance the efficacy of H. pylori eradication and overcome antibiotic resistance, the appropriate use of antibiotics is imperative [3,5ŌĆō7].
Hence, the H. pylori Working Group (Maastricht V) has proposed a nonbismuth quadruple therapy, administered as either sequential or concomitant therapy, as a first-line regimen in areas with high CLA resistance. One of the most successful alternatives for first-line treatment is combinatorial treatment with PPI, AMX, MET, and CLA for 10ŌĆō14 days [1]. Clinical trials and meta-analyses have shown that this therapy cures > 90% of the patients, even in areas with moderately high CLA resistance [8ŌĆō11]. On the basis of these results, concomitant therapy has been widely recommended [1,12].
Acid suppression in eradication therapy has been attributed to the antibacterial activity of PPIs either directly or through the inhibition of urease activity or increased antibiotic stability and activity [13,14]. Recently, a novel potassium-competitive acid blocker (PCAB) has been used to improve the H. pylori eradication rate [14ŌĆō16]. Similar to PPIs, PCAB inhibits gastric H+ and K+-ATPase, an enzyme that catalyzes the final step in the gastric acid secretion pathway [17], showing a more rapid and sustained acid-inhibitory effect than PPIs. Studies comparing the eradication rates between triple therapies based on vonoprazan, an oral PCAB developed in Japan, and PPIs have reported that vonoprazan-based therapies may be an appropriate alternative treatment option for H. pylori infection [18]. Vonoprazan-based triple therapy has shown a higher eradication rate than the PPI-based triple therapy (87.9% vs. 72.8%) in a meta-analysis [16].
Another meta-analysis that included only randomized controlled trials (RCTs) has shown that triple therapy using vonoprazan as an alternative to PPI had a significantly higher eradication rate (91.4% vs. 74.8%) and fewer adverse reactions (32.7% vs. 40.5%) [19]. However, to date, no study on the efficacy of vonoprazan-based concomitant therapy has been conducted.
Tegoprazan has been approved in South Korea for treating erosive reflux disease, nonerosive reflux disease, and gastric ulcers and for eradicating H. pylori. Several experimental and clinical studies have found it to be a potent and highly selective PCAB that showed rapid response from the time of initial administration and also sustained acid suppression [20,21]. The effects of tegoprazan on an intragastric pH of Ōēź4 holding time on days 1 and 7 were similar to those of vonoprazan; the enzyme CYP3A4 metabolizes this drug in humans [21,22]. One study has shown that tegoprazan (50-mg dose) was not less effective than lansoprazole (30-mg dose) in terms of its acid-inhibitory effects in gastric ulcers [21,23] and esomeprazole (40-mg dose) in terms of its healing rates in erosive esophagitis and tolerability [24].
Thus, a tegoprazan-based concomitant regimen is expected to be a robust approach to H. pylori eradication regardless of antibiotic resistance and drug metabolism. Therefore, in this study, we evaluated the efficacy of a 10-day tegoprazan-based concomitant therapy as a first-line treatment for H. pylori eradication.
We conducted a prospective, open-label, single-arm trial at Kyungpook National University Chilgok Hospital, a tertiary center in Daegu, Korea, between December 2020 and September 2021 (cris.nih.go.kr; identifier KCT0005202). The study protocol was approved by the Institutional Review Board of the study site (CHKNUH 2020-03-027). This study was conducted as per the Declaration of Helsinki. We included patients with endoscopically confirmed gastric or duodenal ulcers (including scars), gastritis, gastric dysplasia, or early gastric cancer and those who tested positive for H. pylori. We excluded (1) patients with a history of H. pylori eradication; (2) those with a history of gastrectomy; (3) those with severe concurrent disease or malignancy; (4)those who were pregnant or lactating; (5) those with a history of alcohol abuse or drug addiction; (6) those with a history of allergic reaction to the study drugs; (7) those using antipsychotics, antidepressants, or anxiolytics; and (8) those using PPIs, H2 blockers, antacids, bismuth-containing drugs, or antibiotics within 14ŌĆō28 days before screening. Fig. 1 shows the study protocol. Written informed consent was acquired from all participants before study enrollment.
At the start of the study, we recorded the patientsŌĆÖ demographic and clinical data, including their medical and medication history and any previous H. pylori eradication therapy and confirmed patient eligibility. On visit 1, we performed a physical examination, vital sign assessment, clinical laboratory tests (serum chemistry, H. pylori immunoglobulin G [IgG] tests), and endoscopy. We also conducted biopsy, rapid urease testing (RUT), and antimicrobial susceptibility testing (standard agar plate dilution method) during endoscopy.
On visit 2, we determined the participantŌĆÖs eligibility for this study after assessing their vital signs and current medication history and performing a physical examination and pregnancy test, where appropriate. We administered antimicrobial drugs after confirming the presence of active H. pylori infection.
On visit 3, we determined H. pylori eradication using 13C-urea breath tests with 100-mg UBIT tablets (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) using a cut-off of 2.5%. We monitored serious adverse events (SAEs), acute drug reactions (ADRs), and concomitant medications throughout the study.
The primary endpoint of this study was the H. pylori eradication rate after initiating first-line therapy; the secondary endpoint was the eradication rate on the basis of AMZ, CLA, and MET resistance.
We determined active H. pylori infection using RUT (ARJ Medical, Oldsmar, FL, USA), serum H. pylori IgG test, and histopathology. For RUT, we evaluated the gastric antral and body biopsy specimens separately. We monitored all RUTs for color changes for up to 24 hours. We diagnosed patients with two of the three tests yielding positive results as having H. pylori infection and patients for which all three tests were negative as not having H. pylori infection.
We subjected one gastric mucosal biopsy sample to the direct loop-mediated isothermal amplification (LAMP) assay using the Isopollo┬« H. pylori & ClaR Kit and the Maxpress┬« DNA Kit HS2 (Monitor Inc., Daegu, Korea) according to the manufacturerŌĆÖs protocol [25].
We acquired independently each of the two antral and body biopsy specimens for microbiological culture, after which we determined minimum inhibitory concentrations (MICs) for each isolate colony [26]. We based the susceptibility testing reference on the EUCAST recommendations (AMX, Ōēź 0.5 ╬╝g/mL; CLA, > 0.5 ╬╝g/mL; MET, > 8 ╬╝g/mL; tetracycline, > 4 ╬╝g/mL; and levofloxacin [LEV], >2 ╬╝g/mL) [27]. If one of the H. pylori strains in the antrum or body exhibited antibiotic resistance, we considered a resistant strain to be harbored. We defined multidrug resistance as resistance to two or more antibiotics on the basis of the MIC results. Furthermore, we classified the level of antibiotic resistance as ŌĆ£lowŌĆØ (MICs from > 0.5 ╬╝g/mL to Ōēż 8 ╬╝g/mL for CLA and from > 8 ╬╝g/mL to Ōēż 32 ╬╝g/mL for MET) and ŌĆ£highŌĆØ (MICs of > 8 ╬╝g/mL for CLA, > 32 mg/L for MET) for evaluating whether the MIC level affected the failure of H. pylori eradication in the current regimen [28].
We assigned all eligible participants to the tegoprazan-based concomitant therapy group: tegoprazan (50 mg), AMX (1,000 mg), CLA (500 mg), and MET (500 mg) administered orally twice daily for 10 days.
No previous studies have determined H. pylori eradication rates after first-line tegoprazan-based concomitant therapies. Considering the more potent acid-inhibitory effects of tegoprazan as compared with PPIs, we hypothesized that tegoprazan-based concomitant therapy would be as effective as first-line PPI-based concomitant therapies for H. pylori eradication. We calculated the sample size on the basis of a nationwide multicenter prospective randomized study conducted in Korea showing that PPI-based concomitant therapy had a cure rate of 90.6% on per-protocol (PP) analysis [11]. We determined the protocol-defined sample size to be 67 participants per treatment group to achieve > 90% power for detecting the noninferiority of tegoprazan over PPI, with a noninferiority margin of 10%, using the Farrington and Manning test [29]. After accounting for possible dropouts (approximately 20% of the participants), we targeted 84 participants for recruitment.
We performed efficacy assessments in the complete analysis set. For the primary endpoints in the treatment group, we calculated frequency, point estimates, and two-sided 95% confidence intervals (CIs). As appropriate, we compared secondary endpoints and demographic data using the chi-squared test or FisherŌĆÖs exact test. We set statistical significance at p < 0.05. We used the Statistical Package for the Social Sciences version 15.0 (SPSS Inc., Chicago, IL, USA) for all analyses. All authors had access to the study data and reviewed and approved the final manuscript.
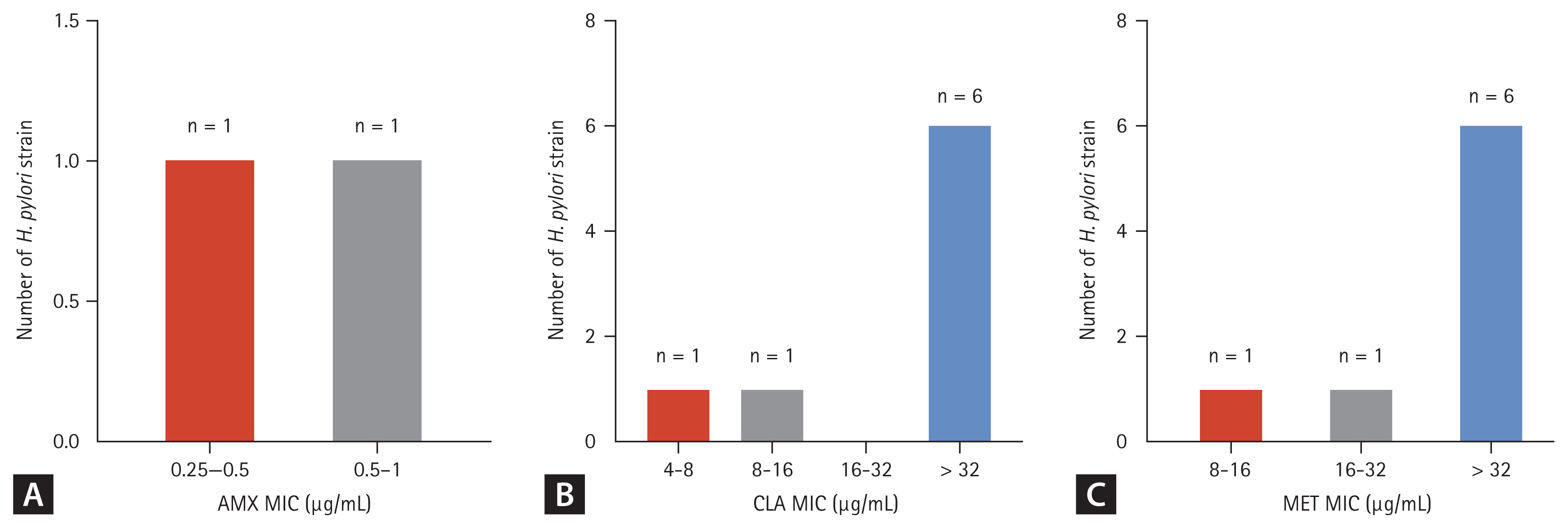
Of the 98 participants assessed for eligibility, we allocated 84 who had provided written informed consent to receive the 10-day tegoprazan-based nonbismuth quadruple therapy (Fig. 2). Ultimately, 79 patients (94.0%) completed the 10-day first-line tegoprazan-based concomitant regimen for H. pylori eradication. We excluded participants who failed to follow the study protocol (two did not complete the eradication regimen; three did not undergo the 13C-urea breath test after completing the regimen), resulting in a treatment compliance rate of 97.6%.
Of the 84 patients, we successfully cultured H. pylori strains from 73 (86.9%) using the agar dilution method. Table 1 summarizes antibiotic susceptibility and other baseline characteristics. Notably, 32 (43.8%) patients had a H. pylori strain resistant to a single antibiotic, and 21 (28.7%) patients had strains resistant to two or more antibiotics. Moreover, 8 of the 73 (11.0%) patients with successful H. pylori cultures exhibited resistance to CLA and MET with or without resistance to other antibiotics (Table 2).
The eradication rates (primary endpoint) were 90.5% (76/84) in the intention-to-treat (ITT) analysis (95% CI, 82.1ŌĆō95.8) and 96.2% (76/79) in the PP analysis (95% CI, 83.4ŌĆō97.6); thus, tegoprazan-based concomitant therapy was not inferior as the lower bound of the CI did not fall beyond the noninferiority margin of 10% (reference rate, 80.6%; p = 0.0110). Table 3 shows the characteristics of the successful and failed groups.
We included all participants who received at least one dose of the medication in the safety analysis. During the eradication phase, the overall incidence of adverse events and drug-related treatment-emergent adverse events (TEAEs) were 53.6% and 51.2%, respectively. The observed TEAEs included urticaria, nasopharyngitis, liver function abnormalities, nausea, vomiting, and diarrhea. Two participants discontinued treatment because of ADR occurrence during the primary regimen. However, no SAEs occurred during the study (Table 4).
LAMPŌĆōpolymerase chain reaction for the gastric samples of 21 participants showed CLA resistance. Fig. 3 illustrates the MIC levels of each antibiotic for the H. pylori strains. Of the 19 CLA-resistant strains, 18 showed a high CLA MIC range (> 8 ╬╝g/mL); 14 of the 32 MET-resistant strains showed a high MET MIC range (> 32 ╬╝g/mL). Fig. 4 presents the MICs of the strains that showed resistance to two or more antibiotics (especially AMX, CLA, and MET). Seven strains showed a high CLA MIC range; six showed a high MET MIC range. The MIC range of the H. pylori specimen from the one patient who failed to respond to the current regimen was 0.25ŌĆō0.5 ╬╝g/mL for AMX, > 32 ╬╝g/mL for CLA, > 32 ╬╝g/mL for MET, and 8ŌĆō16 ╬╝g/mL for LEV. After analyzing the relationship between eradication rate and antibiotic susceptibility in the 73 participants, only 1 patient who exhibited resistance to AMX, CLA, MET, and LEV on the basis of the MIC results failed to respond to the tegoprazan-based concomitant regimen (Table 3). The other two patients who failed to respond to the current regimen had negative H. pylori culture results.
This study evaluated whether tegoprazan is not inferior to PPI-based concomitant therapy for primary eradication of H. pylori infection in terms of efficacy and safety. Notably, our 10-day tegoprazan-based concomitant therapy exhibited substantial primary H. pylori eradication efficacy (90.5% in the ITT analysis and 96.2% in the PP analysis). A previous meta-analysis of 19 clinical trials on conventional PPI-based nonbismuth quadruple therapy, including 2,070 patients with H. pylori infection, has revealed a mean cure rate of 88% (95% CI, 85ŌĆō91%) [30]. RCTs comparing PPI-based concomitant therapy (481 patients) with CLA triple therapy (503 patients) have shown ITT cure rates of 90% and 78%, respectively (OR, 2.36; 95% CI, 1.67ŌĆō3.34) [30]. A previous Korean nationwide multicenter prospective randomized study has shown that 10 days of PPI-based concomitant therapy had a cure rate of 90.6% on PP analysis [11]. Considering the aforementioned favorable evidence, several clinical guidelines have considered concomitant therapy as a promising treatment option owing to the high associated cure rates in international studies [1,5,11,12,31].
As compared with previous PPI-based concomitant regimen studies, we predicted that a 10-day tegoprazan-based concomitant regimen would not have inferior or superior efficacy for primary H. pylori eradication. Furthermore, this regimen might prove beneficial for cases with dual MET-and CLA-resistant strains despite the high CLA and MET MIC values in the study results.
Appropriate inhibition of acid secretion is imperative for increasing the success rate of H. pylori eradication. Several phase I studies in animals have shown that tegoprazan has strong antisecretory potency and rapid action. Several RCTs have shown the drugŌĆÖs efficacy in treating gastroesophageal reflux disease and benign gastric ulcers [20,21,23,24,32ŌĆō35]. Its suppressive effects on acid secretion can reach a maximum within 0.5ŌĆō1.0 hours of administration. Pharmacodynamic data for tegoprazan have indicated that 50- and 100-mg doses can increase intragastric pH to Ōēź4, which is significantly higher than that of PPIs [36].
No study has compared the efficacy of the nonbismuth quadruple regimen with that of another PCAB, vonoprazan, developed in Japan. However, a randomized, double-blind study has shown that 7 days of vonoprazan-based triple therapy resulted in superior efficacy in H. pylori eradication as compared with that of lansoprazole (92.6% vs. 75.9%), and the H. pylori eradication rate in CLA-resistant strains (30.4% for vonoprazan vs. 35.9% for lansoprazole) was significantly higher with vonoprazan-based triple therapy (82.0%) than that with lansoprazole-based triple therapy (40.0%) in a subgroup analysis (p < 0.0001) [14]. The mechanism by which PCAB appears to overcome CLA resistance is unknown.
In vitro, lansoprazole and omeprazole have been shown to have specific H. pylori antimicrobial properties as determined by the agar dilution method at neutral pH [37]. In addition, H. pylori isolates with CLA (46.3%) and MET (55.6%) resistance have demonstrated improvement in MIC values following tegoprazan administration. Furthermore, tegoprazan has demonstrated a more frequent susceptibility acquisition with MET than vonoprazan (20.6% vs. 4.7%, p = 0.014) [38]. A previous clinical study has shown that the coadministration of tegoprazan with AMX and CLA caused a moderate increase in the Cssmax (2.2-fold) and AUCŽä (2.7-fold) of tegoprazan as compared with that of the administration of tegoprazan alone [39]. This result predicted that an eradication regimen containing AMX and CLA with tegoprazan could increase the pharmacokinetic exposure to tegoprazan and the metabolites of tegoprazan and CLA, without influencing the pharmacokinetic changes in the exposure to AMX or CLA. Furthermore, the pharmacodynamic properties of tegoprazan suggest that tegoprazan-based therapy is superior in the rapid attainment of therapeutically effective pH values from day 1 of treatment and in the sustainability of the achieved pH levels throughout a 24-h period [39].
These favorable outcomes suggest that tegoprazan-containing eradication therapy may increase anti-H. pylori effects, affect antibiotic resistance mechanisms, and decrease gastric acid efficacy. In addition, the tegoprazan-based concomitant therapy was generally well tolerated, with only two participants failing to complete the 10-day regimen and no drug-related SAEs being observed. However, in a recent phase III clinical trial, which used tegoprazan as a part of a first-line triple therapy regimen in Korea, the H. pylori eradication rates in the tegoprazan and lansoprazole groups were 62.86% (110/175) and 60.57% (106/175) in an ITT analysis and 69.33% (104/150) and 67.33% (101/150) in a PP analysis (noninferiority test, p = 0.009 and p = 0.013), respectively [40]. This study showed that tegoprazan-based triple therapy did not overcome the CLA resistance of H. pylori in Korea.
On the contrary, our study showed that tegoprazan-based concomitant therapy can be as effective as PPI-based concomitant therapy. Our results indicate that a concomitant regimen can be more effective for overcoming CLA and MET resistance, and MET resistance is relatively low in Korea.
However, our study has some limitations, with the most important one being that it was a primitive study and not a blinded RCT comparing tegoprazan-based therapy with PPI- or vonoprazan-containing concomitant therapy. No H. pylori efficacy data for tegoprazan were available. However, a preplanned blinded interim analysis was included in the protocol to ensure that a sufficient number of participants were recruited and that the desired statistical power was achieved. Another limitation was that we failed to show the MIC results for the two patients who failed this regimen, although both showed CLA resistance on the LAMP.
In conclusion, the 10-day tegoprazan-based concomitant therapy was as effective as a PPI-based concomitant therapy in H. pylori eradication and was well tolerated. Therefore, this regimen can be an effective first-line therapy for overcoming antibiotic resistance.
1. The eradication rates of a 10-day tegoprazan-based (50-mg dose) concomitant therapy as a first-line treatment for H. pylori were 90.5% and 96.2% on ITT and PP analyses, respectively.
2. A 10-day tegoprazan-based concomitant regimen may prove beneficial for patients with dual metronidazole (MET)/clarithromycin (CLA)-resistant strains, despite high CLA and MET MIC values.
3. Given the increasing resistance to CLA and MET and the declining rates of clinical response to PPI-based triple therapy, which have become a globally challenging issue, a tegoprazan-based concomitant regimen may be more effective for H. pylori eradication.
Notes
Figure┬Ā1
Study flowchart. EGD, endoscopy; H. pylori, Helicobacter pylori; IgG, immunoglobulin G; RUT, rapid urease test; LAMP, loop-mediated isothermal amplification; MIC, minimum inhibitory concentration; TACM, 10 days of a concomitant regimen containing tegoprazan, amoxicillin, clarithromycin, and metronidazole; SAE, serious adverse event; ADR, acute drug reaction; UBT, 13C-urea breath test; ITT, intention-to-treat; PP, per-protocol.

Figure┬Ā2
Study flow diagram showing trial progression. H. pylori, Helicobacter pylori; MIC, minimum inhibitory concentration; UBT, 13C-urea breath test.
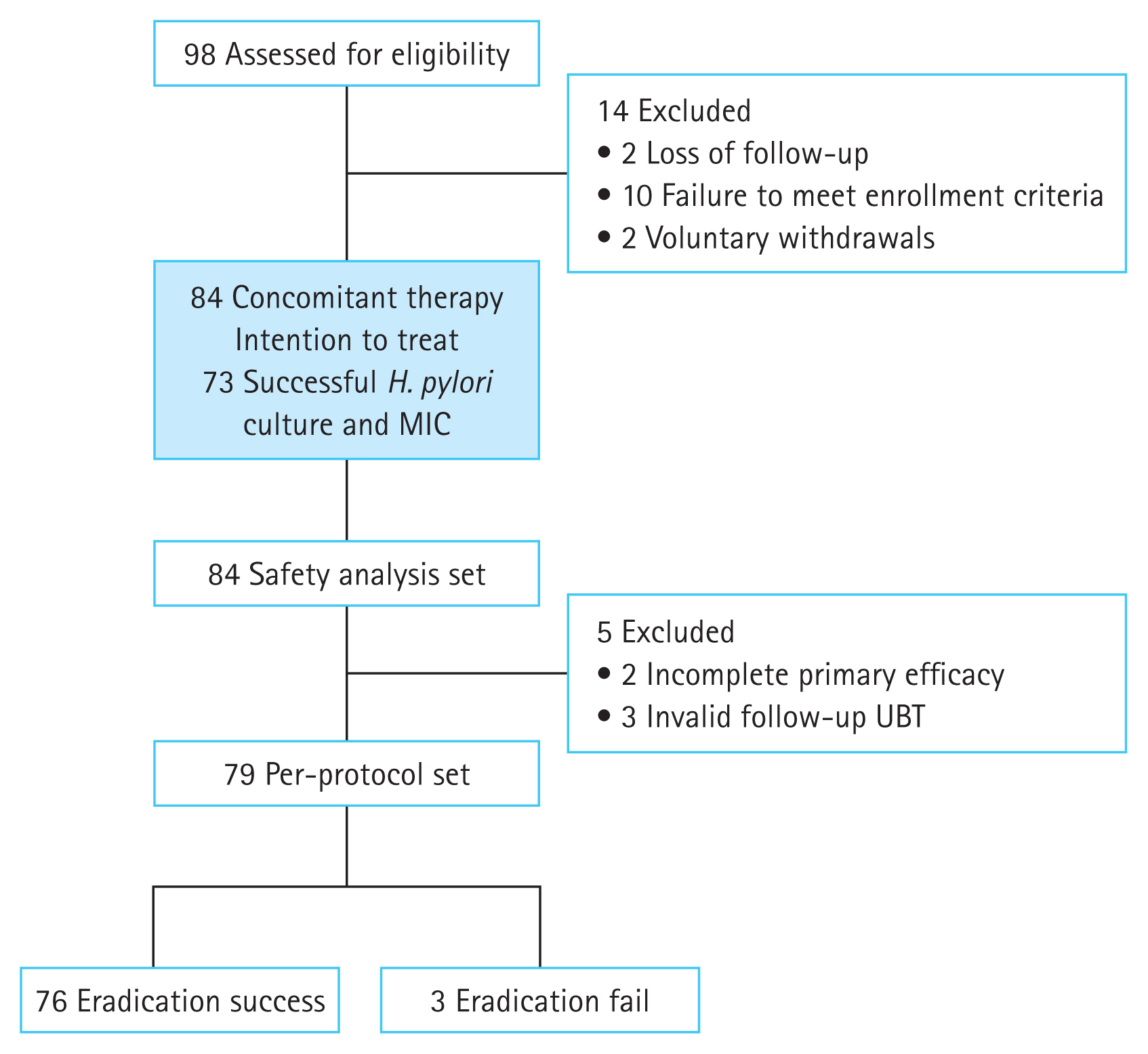
Figure┬Ā3
H. pylori culture and MICs of antibiotics. (A) AMX, (B) CLA, (C) MET, (D) LEV. H. pylori, Helicobacter pylori; AMX, amoxicillin; CLA, clarithromycin; MET, metronidazole; LEV, levofloxacin; MIC, minimum inhibitory concentration.

Figure┬Ā4
H. pylori culture and MICs of two or more antibiotics resistance. (A) AMX, (B) CLA, (C) MET. H. pylori, Helicobacter pylori; AMX, amoxicillin; CLA, clarithromycin; MET, metronidazole; MIC, minimum inhibitory concentration.
Table┬Ā1
Demographic and baseline characteristics
Table┬Ā2
Antibiotic resistance based on Helicobacter pylori culture and MIC test
Table┬Ā3
Baseline characteristics and MIC results of successful and failed eradication groups
| Characteristic | Successful eradication group (n = 76) | Failed eradication group (n = 8) | p value |
|---|---|---|---|
| Age, yr | 59.9 ┬▒ 10.3 | 58.2 ┬▒ 10.0 | 0.574 |
| Sex, male | 52 (68.4) | 4 (50.0) | |
| Diagnosis | 0.721 | ||
| ŌĆāChronic atrophic gastritis | 29 (38.2) | 2 (25.0) | |
| ŌĆāPeptic ulcer | 15 (19.7) | 1 (12.5) | |
| ŌĆāGastric adenoma | 17 (22.4) | 3 (37.5) | |
| ŌĆāEarly gastric cancer | 15 (19.7) | 2 (25.0) | |
| Helicobacter pylori MIC results (n = 73) | 67 | 6 | N/A |
| ŌĆāNo antibiotic resistance | 19 (28.6) | 1 (16.7) | |
| ŌĆāAMX | 1 (1.4) | 0 (0.0) | |
| ŌĆāCLA | 4 (6.0) | 1 (16.7) | |
| ŌĆāMET | 16 (23.9) | 1 (16.7) | |
| ŌĆāLEV | 9 (13.4) | 0 (0.0) | |
| ŌĆāAMXŌĆōCLA | 1 (1.4) | 0 (0.0) | |
| ŌĆāAMXŌĆōMET | 2 (3.0) | 0 (0.0) | |
| ŌĆāCLAŌĆōMET | 3 (4.5) | 0 (0.0) | |
| ŌĆāCLAŌĆōLEV | 4 (6.0) | 0 (0.0) | |
| ŌĆāMETŌĆōLEV | 4 (6.0) | 0 (0.0) | |
| ŌĆāAMXŌĆōCLAŌĆōMET | 0 (0.0) | 1 (16.7) | |
| ŌĆāAMXŌĆōMETŌĆōLEV | 1 (1.4) | 0 (0.0) | |
| ŌĆāCLAŌĆōMETŌĆōLEV | 2 (3.0) | 1 (16.6) | |
| ŌĆāCLAŌĆōTCŌĆōLEV | 1 (1.4) | 0 (0.0) | |
| ŌĆāAMXŌĆōCLAŌĆōMETŌĆōLEV | 0 (0.0) | 1 (16.6)a) |
Table┬Ā4
Summary of TEAEs
REFERENCES
1. Malfertheiner P, Megraud F, OŌĆÖMorain CA, et al. European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017;66:6ŌĆō30.


2. Boyanova L, Hadzhiyski P, Kandilarov N, Markovska R, Mitov I. Multidrug resistance in Helicobacter pylori: current state and future directions. Expert Rev Clin Pharmacol 2019;12:909ŌĆō15.


3. Gatta L, Scarpignato C, Fiorini G, et al. Impact of primary antibiotic resistance on the effectiveness of sequential therapy for Helicobacter pylori infection: lessons from a 5-year study on a large number of strains. Aliment Pharmacol Ther 2018;47:1261ŌĆō9.



4. Lee JH, Ahn JY, Choi KD, et al. Korean College of Helicobacter; Upper Gastrointestinal Research. Nationwide antibiotic resistance mapping of Helicobacter pylori in Korea: a prospective multicenter study. Helicobacter 2019;24:e12592.



5. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol 2017;112:212ŌĆō39.



6. Fallone CA, Moss SF, Malfertheiner P. Reconciliation of recent Helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics. Gastroenterology 2019;157:44ŌĆō53.


7. Mu├▒oz N, S├Īnchez-Delgado J, Baylina M, L├│pez-G├│ngora S, Calvet X. Prevalence of Helicobacter pylori resistance after failure of first-line therapy. A systematic review. Gastroenterol Hepatol 2018;41:654ŌĆō62.


8. Liou JM, Fang YJ, Chen CC, et al. Taiwan Gastrointestinal Disease and Helicobacter Consortium. Concomitant, bismuth quadruple, and 14-day triple therapy in the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet 2016;388:2355ŌĆō65.


9. Molina-Infante J, Lucendo AJ, Angueira T, et al. European Registry on H. pylori management (Hp-EuReg). Optimised empiric triple and concomitant therapy for Helicobacter pylori eradication in clinical practice: the OPTRICON study. Aliment Pharmacol Ther 2015;41:581ŌĆō9.


10. Molina-Infante J, Romano M, Fernandez-Bermejo M, et al. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology 2013;145:121ŌĆō8e1.


11. Kim BJ, Lee H, Lee YC, et al. Korean College of Helicobacter Upper Gastrointestinal Research. Ten-day concomitant, 10-day sequential, and 7-day triple therapy as first-line treatment for Helicobacter pylori infection: a nationwide randomized trial in Korea. Gut Liver 2019;13:531ŌĆō40.



12. Fallone CA, Chiba N, van Zanten SV, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016;151:51ŌĆō69e14.


13. Scott DR, Sachs G, Marcus EA. The role of acid inhibition in Helicobacter pylori eradication. F1000Res 2016;5:F1000 Faculty Rev-1747.



14. Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut 2016;65:1439ŌĆō46.



15. Kiyotoki S, Nishikawa J, Sakaida I. Efficacy of vonoprazan for Helicobacter pylori eradication. Intern Med 2020;59:153ŌĆō61.



16. Jung YS, Kim EH, Park CH. Systematic review with meta-analysis: the efficacy of vonoprazan-based triple therapy on Helicobacter pylori eradication. Aliment Pharmacol Ther 2017;46:106ŌĆō14.



17. Shin JM, Inatomi N, Munson K, et al. Characterization of a novel potassium-competitive acid blocker of the gastric H,K-ATPase, 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438). J Pharmacol Exp Ther 2011;339:412ŌĆō20.



18. Rokkas T, Gisbert JP, Malfertheiner P, et al. Comparative effectiveness of multiple different first-line treatment regimens for Helicobacter pylori infection: a network meta-analysis. Gastroenterology 2021;161:495ŌĆō507e4.


19. Lyu QJ, Pu QH, Zhong XF, Zhang J. Efficacy and safety of vonoprazan-based versus proton pump inhibitor-based triple therapy for Helicobacter pylori eradication: a meta-analysis of randomized clinical trials. Biomed Res Int 2019;2019:9781212.




20. Takahashi N, Take Y. Tegoprazan, a novel potassium-competitive acid blocker to control gastric acid secretion and motility. J Pharmacol Exp Ther 2018;364:275ŌĆō86.


21. Han S, Choi HY, Kim YH, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple oral doses of tegoprazan (CJ-12420), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther 2019;50:751ŌĆō9.



22. Kagami T, Sahara S, Ichikawa H, et al. Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment Pharmacol Ther 2016;43:1048ŌĆō59.


23. Cho YK, Choi MG, Choi SC, et al. Randomised clinical trial: tegoprazan, a novel potassium-competitive acid blocker, or lansoprazole in the treatment of gastric ulcer. Aliment Pharmacol Ther 2020;52:789ŌĆō97.




24. Lee KJ, Son BK, Kim GH, et al. Randomised phase 3 trial: tegoprazan, a novel potassium-competitive acid blocker, vs. esomeprazole in patients with erosive oesophagitis. Aliment Pharmacol Ther 2019;49:864ŌĆō72.




25. Park CG, Kim S, Jeon HS, Han S. Validation of loop-mediated isothermal amplification to detect Helicobacter pylori and 23S rRNA mutations: a prospective, observational clinical cohort study. J Clin Lab Anal 2021;35:e23563.




26. Kwon YH, Jeon SW, Nam SY, Lee HS, Park JH. Efficacy of tailored therapy for Helicobacter pylori eradication based on clarithromycin resistance and survey of previous antibiotic exposure: a single-center prospective pilot study. Helicobacter 2019;24:e12585.



27. EUCAST. Clinical breakpoints - breakpoints and guidance, version 13.0 [Internet]. V├żxj├Č: EUCAST, c2023. [cited 2023 Jan. 2]. Available from: https://www.eucast.org/clinical_breakpoints
.
28. Hwang JY, Kim C, Kwon YH, et al. Clarithromycin and metronidazole resistance is the main cause of failure in ultimate Helicobacter pylori eradication. Dig Dis 2021;39:451ŌĆō61.



29. Roebruck P, K├╝hn A. Comparison of tests and sample size formulae for proving therapeutic equivalence based on the difference of binomial probabilities. Stat Med 1995;14:1583ŌĆō94.


30. Gisbert JP, Calvet X. Update on non-bismuth quadruple (concomitant) therapy for eradication of Helicobacter pylori. Clin Exp Gastroenterol 2012;5:23ŌĆō34.



31. Jung HK, Kang SJ, Lee YC, et al. Korean College of Helicobacter and Upper Gastrointestinal Research. Evidence-based guidelines for the treatment of Helicobacter pylori infection in Korea 2020. Gut Liver 2021;15:168ŌĆō95.


32. Han S, Choi HY, Kim YH, et al. Effect of food on the pharmacokinetics and pharmacodynamics of a single oral dose of tegoprazan. Clin Ther 2021;43:1371ŌĆō80.


33. Kim DK, Lee KH, Kim SJ, et al. Effects of tegoprazan, a novel potassium-competitive acid blocker, on rat models of gastric acid-related disease. J Pharmacol Exp Ther 2019;369:318ŌĆō27.


34. Abdel-Aziz Y, Metz DC, Howden CW. Review article: potassium-competitive acid blockers for the treatment of acid-related disorders. Aliment Pharmacol Ther 2021;53:794ŌĆō809.


35. Kim SH, Cho KB, Chun HJ, et al. Randomised clinical trial: comparison of tegoprazan and placebo in non-erosive reflux disease. Aliment Pharmacol Ther 2021;54:402ŌĆō11.




36. Yang E, Kim S, Kim B, et al. Night-time gastric acid suppression by tegoprazan compared to vonoprazan or esomeprazole. Br J Clin Pharmacol 2022;88:3288ŌĆō96.




37. Iwahi T, Satoh H, Nakao M, et al. Lansoprazole, a novel benzimidazole proton pump inhibitor, and its related compounds have selective activity against Helicobacter pylori. Antimicrob Agents Chemother 1991;35:490ŌĆō6.




38. Lee JW, Kim N, Nam RH, et al. Efficacy of tegoprazan for improving the susceptibility of antimicrobial agents against antibiotic-resistant Helicobacter pylori. Gut Liver 2021;15:53ŌĆō60.



-
METRICS

-
- 0 Crossref
- 1 Scopus
- 289 View
- 244 Download
- Related articles
-
The antibacterial effect of fatty acids on Helicobacter pylori infection2016 January;31(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


