 |
 |
| Korean J Intern Med > Volume 38(3); 2023 > Article |
|
See editorial "Ankle brachial index: a simple path to the future" on page 277.
Abstract
Background/Aims
Bleeding events after percutaneous coronary intervention (PCI) have important prognostic implications. Data on the influence of an abnormal ankle-brachial index (ABI) on both ischemic and bleeding events in patients undergoing PCI are limited.
Methods
We included patients who underwent PCI with available ABI data (abnormal ABI, ≤ 0.9 or > 1.4). The primary endpoint was the composite of all-cause death, myocardial infarction (MI), stroke, and major bleeding.
Results
Among 4,747 patients, an abnormal ABI was observed in 610 patients (12.9%). During follow-up (median, 31 months), the 5-year cumulative incidence of adverse clinical events was higher in the abnormal ABI group than in the normal ABI group: primary endpoint (36.0% vs. 14.5%, log-rank test, p < 0.001); all-cause death (19.4% vs. 5.1%, log-rank test, p < 0.001); MI (6.3% vs. 4.1%, log-rank test, p = 0.013); stroke (6.2% vs. 2.7%, log-rank test, p = 0.001); and major bleeding (8.9% vs. 3.7%, log-rank test, p < 0.001). An abnormal ABI was an independent risk factor for all-cause death (hazard ratio [HR], 3.05; p < 0.001), stroke (HR, 1.79; p = 0.042), and major bleeding (HR, 1.61; p = 0.034).
Peripheral artery disease (PAD) is associated with systemic atherosclerosis and is one of the leading causes of atherosclerotic cardiovascular (CV) morbidity and mortality [1]. Among patients with coronary artery disease (CAD), approximately 13% to 22% have PAD, which increases the risk for future ischemic events [2,3]. Likely CAD, PAD is managed through lifestyle modifications, medical treatment, endovascular repair, or surgery. Of these, lifestyle modifications and pharmacological treatment are recommended to improve clinical outcomes [4]. The diagnostic approach for PAD is important because risk stratification may provide evidence for determining the manner and intensity of treatment [5,6]. In general, patients with both CAD and PAD require more intensive medical management, including potent antithrombotic or lipid-lowering therapy, for secondary prevention [7-9]. Recently, interest in the role of PAD and the prognosis of bleeding events in patients with CAD has increased. The COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial suggested that low-dose novel oral anticoagulants as an adjunct to aspirin compared with aspirin alone reduce ischemic events but increase major bleeding events in patients with PAD [10].
The ankle-brachial index (ABI) is a well-established modality for assessing PAD, and current guidelines recommend specific criteria for an abnormal ABI (ABI of ≤ 0.9 or > 1.4) [11,12]. Earlier research has reported that an abnormal ABI was associated with an increased risk of ischemic events [13,14]. However, an abnormal ABI has not been well investigated as a risk factor for ischemic and bleeding events in patients undergoing percutaneous coronary intervention (PCI). Therefore, we performed this study to evaluate the influence of an abnormal ABI on both ischemic and bleeding events in patients undergoing PCI.
A total of 5,160 patients who underwent PCI at the Gyeongsang National University Hospital between January 2011 and December 2016 were enrolled (Fig. 1). The exclusion criteria were as follows: 1) prior evidence of PAD treatment (n = 105), 2) no ABI measurement (n = 138), 3) use of oral anticoagulants at discharge (n = 87), and 4) missing follow-up data after discharge (n = 83). Finally, 4,747 patients were included in the study. Clinical characteristics, presentation, angiographic and procedural findings, discharge medication, and clinical outcome data were prospectively collected by the research coordinators. The patients were routinely followed up at 1, 6, and 12 months after the index procedure and annually thereafter. Further information was collected from medical records or telephone interviews, when necessary. The Institutional Review Board of Gyeongsang National University Hospital approved the study protocol (No. GNUH 2018-07-012) and waived the requirement for written informed consent for access to an institutional registry. The study was performed in accordance with the Good Clinical Practice Guidelines and the principles of the Declaration of Helsinki.
Using a Doppler ultrasound device (VP-1000; Colin Co., Ltd., Komake, Japan), we measured the ABI for each leg before PCI (in emergent cases, before discharge). The sequence of limb pressure measurements was as follows: the first arm, first posterior tibial artery, first dorsalis pedis artery, second posterior tibial artery, second dorsalis pedis artery, and second arm. Each pressure was measured twice, and the average of each pressure was used in the calculations. The ABI of each leg was calculated by dividing the posterior tibial or dorsalis pedis pressure, whichever was higher, by the systolic blood pressure of the right or left arm, whichever was higher. The lowest ABI in the left and right legs was selected. The ABI threshold for detecting PAD was ≤ 0.90, showing > 80% sensitivity and > 90% specificity in earlier studies; an ABI of > 1.40 was defined as abnormal, predicting the incidence of PAD with 60% to 80% accuracy [15,16].
The primary endpoint was the composite of adverse clinical events, including all-cause death, myocardial infarction (MI), stroke, and major bleeding. All endpoints were described according to the Academic Research Consortium (ARC) definitions [17,18]. The individual components of the primary endpoint were analyzed as the secondary endpoints. All-cause death included death from cardiac and non-cardiac causes during follow-up. We also evaluated deaths due to fatal bleeding. MI was defined as increased cardiac troponin values with ischemic symptoms or ischemic changes on electrocardiography or imaging evidence of recent loss of viable myocardium or a new regional wall motion abnormality. Stroke, indicated by a rapid onset of a focal or global neurological deficit with signs or symptoms, was confirmed by a neurologist based on the neuroimaging results. Major bleeding was defined as Bleeding ARC type 3 or 5 bleeding.
The Kolmogorov-Smirnov test was performed to analyze the normal distribution of continuous variables. Continuous variables were presented as means ± standard deviations or as medians (interquartile ranges [IQRs]), as appropriate, and categorical variables as frequencies and percentages. Student’s unpaired t-test was used for parametric continuous variables and the Mann-Whitney U test for non-parametric continuous variables. Categorical variables were compared using Pearson’s chi-square test or Fisher’s exact test, as appropriate. Receiver-operating characteristic (ROC) curve analysis was performed to find optimal cutoffs of continuous variables, which then were changed into the dichotomous covariates. We compared the area under curve (AUC) and calculated discrimination improvement using identified risk factors of adverse clinical events.
All demographic characteristics and laboratory measurements were evaluated using a univariate analysis to predict adverse clinical events. Variables with a p value of < 0.1 in the univariate analysis were then entered into the multivariate Cox proportional hazard analysis to identify independent correlates of ischemic and bleeding events. According to the ABI, survival curves were constructed using Kaplan-Meier estimates and compared using the log-rank test. A p value of < 0.05 was considered statistically significant, and all statistical analyses were performed using SPSS version 24.0 (SPSS Inc., Chicago, IL, USA) and Medcalc version 13.3.3.0 statistical software (Medcalc, Ostend, Belgium).
Among the 4,747 patients, an abnormal ABI was observed in 610 patients (12.9%) (594 patients with an ABI of ≤ 0.9 and 16 patients with an ABI of > 1.4). Compared with the normal ABI group, the abnormal ABI group showed unique clinical characteristics, including older age, lower body mass index, hypertension, diabetes, prior ischemic stroke, and chronic kidney disease (CKD) (Table 1). The clinical presentation of acute coronary syndrome (ACS), especially acute MI, was associated with a higher rate of an abnormal ABI. The abnormal ABI group had a higher white blood cell (WBC) count and high-sensitivity C-reactive protein (hs-CRP) level than the normal ABI group. The incidence of anemia, renal dysfunction, and reduced left ventricle (LV) function was also higher in the abnormal ABI group than in the normal ABI group. The angiographic and procedural findings showed that multivessel CAD occurred more frequently and that PCI was performed more commonly in the abnormal ABI group than in the normal ABI group.
The median follow-up time was 31.0 months (IQR, 15.3–49.7). The number of events estimated cumulative incidence rate based on Kaplan-Meier curve were following; 211 all-cause deaths, 170 MIs, 112 strokes, and 153 major bleeding events. The median ABI of the entire population was 1.07 (IQR, 0.99–1.13). The ABI was significantly lower for each composite of clinical outcome (primary endpoint, 0.96 ± 0.19 vs. 1.04 ± 0.13, p < 0.001; all-cause death, 0.91 ± 0.20 vs. 1.04 ± 0.13, p < 0.001; MI, 1.00 ± 0.17 vs. 1.04 ± 0.14, p = 0.011; stroke, 0.99 ± 0.16 vs. 1.04 ± 0.14, p = 0.010; major bleeding, 0.98 ± 0.18 vs. 1.04 ± 0.14, p = 0.001).
The number of adverse clinical events increased steadily throughout the follow-up period (Fig. 2). The Kaplan-Meier curves showed an increasing divergence between the two groups. The abnormal ABI group had a higher 5-year cumulative incidence of the primary endpoint (36.0% vs.14.5%, log-rank test, p < 0.001), all-cause death (19.4% vs. 5.1%, log-rank test, p < 0.001), MI (6.3% vs. 4.1%, log-rank test, p = 0.013), stroke (6.2% vs. 2.7%, log-rank test, p = 0.001), and major bleeding (8.9% vs. 3.7%, log-rank test, p < 0.001) (Fig. 2). In the multivariate analysis, an abnormal ABI was found as an independent risk factor for the primary endpoint (hazard ratio [HR], 2.21; 95% confidence interval [CI], 1.74–2.80; p < 0.001), all-cause death (HR, 3.05; 95% CI, 2.17–4.31; p < 0.001), stroke (HR, 1.79; 95% CI, 1.02–3.14; p = 0.042), and major bleeding (HR, 1.61; 95% CI, 1.03–2.51; p = 0.034) (Table 2). In predicting a primary endpoint, an abnormal ABI increased predictive value (AUC 0.674 vs. AUC 0.656, p < 0.001) compared to reference variables (age > 65 years, diabetes, ACS, previous PCI, LV ejection fraction < 50%, renal dysfunction, and anemia) (Fig. 3). Combination of abnormal ABI and reference variables (dyslipidemia, renal dysfunction, and anemia) had more powerful predictive value for major bleeding (AUC 0.597 vs. AUC 0.569, p = 0.014).
This study reports the long-term (5-year) influence of an abnormal ABI on both ischemic and bleeding events in patients undergoing PCI. The main findings were as follows: 1) approximately one eighth of the patients (12.9%) who underwent PCI had a newly identified abnormal ABI (ABI of ≤ 0.9 or > 1.4); 2) compared with the normal ABI group, the abnormal ABI group had known baseline risk factors for CV and bleeding events; 3) an abnormal ABI was associated with worse clinical outcomes, including major bleeding events after PCI; and 4) an abnormal ABI was an independent risk factor for the primary endpoint (HR, 2.21), all-cause death (HR, 3.05), non-fatal stroke (HR, 1.79), and major bleeding (HR, 1.61).
CV disease is the leading cause of mortality and morbidity worldwide. The prognosis worsens as the number of arterial disease locations increases. Patients with PAD have shown more widespread atherosclerosis, which is related to an increase in the number of ischemic events, including CV death [7,8]. In this study, an abnormal ABI was observed in 12.9% of the patients, and the abnormal ABI group had a higher 5-year incidence of all-cause death (19.4% vs. 5.1%, log-rank test, p < 0.001) with more than a threefold hazard risk, which is consistent with earlier data [19,20]. The 5-year incidence of the primary endpoint and major bleeding events was higher in the abnormal ABI group than in the normal ABI group (36.0% vs.14.5%, log-rank test, p < 0.001; 8.9% vs. 3.7%, log-rank test, p < 0.001, respectively). Nakahashi et al. [21] reported a significantly higher 30-day incidence of major bleeding events in patients with a decreased ABI than in those with a normal ABI (21.9% vs. 6.0%, p < 0.001). Although it is thought that the bleeding event rate in their study was higher owing to the different clinical conditions (e.g., rate of oral anticoagulant use, femoral approach, mechanical cardiac support, or presence of ACS), the clinical insights are in line with those of our study.
Bleeding events in patients undergoing PCI have important prognostic implications. Patients with a high bleeding risk (HBR) should be assessed in terms of their thrombotic risk [22]. Anemia (moderate or severe), oral anticoagulation, malignancy, end-stage CKD, planned surgery, and thrombocytopenia have recently been proposed as the major ARC-HBR criteria (by prevalence order) [23]. Age of > 75 years, moderate CKD, anemia (mild), prior stroke, and prior bleeding were minor ARC-HBR criteria. An HBR is more frequently observed among East Asians, and relevant evidence suggests the optimization of pharmacologic therapy for patients with an HBR [24,25]. Sotomi et al. [26] reported a practical method for assessing the tradeoff between thrombotic and bleeding risks according to the ARC-HBR criteria. In our study, anemia (HR, 1.84), renal dysfunction (HR, 1.64), dyslipidemia (HR, 1.61), and an abnormal ABI (HR, 1.61) were significant predictors of major bleeding after PCI. Based on these data, it would be reasonable to include abnormal ABIs in the risk stratification for bleeding.
This study showed that an asymptomatic newly identified abnormal ABI was associated with long-term ischemic and bleeding events in patients undergoing PCI. The prevalence of ACS, increased levels of inflammatory biomarkers (WBC count and hs-CRP level), anemia, renal dysfunction, LV dysfunction, and multivessel CAD or PCI was higher in the abnormal ABI group than in the normal ABI group. These variables may contribute to worse clinical outcomes [27,28]. Prior data showed that an abnormal ABI might cause critical limb ischemia, which could be related to increased all-cause mortality [14,29]. In our study, the all-cause death rate was 4.0% (194 cases) in the total population. Of these cases, more than half (54.1%) were from cardiac causes and almost a tenth (9.8%) from bleeding events. This implies that not only CV morbidity, but also major bleeding can be an important cause of death after PCI. Accordingly, an abnormal ABI influences bleeding events and all-cause death after PCI [21]. Given these perspectives, patients with PAD are at a high risk for future ischemic and bleeding events, and screening and stratifying the risk in patients undergoing PCI would be reasonable.
The role of PAD and the prognosis of major bleeding events in patients with CAD have recently been highlighted [10]. The goal of secondary prevention strategies using antithrombotics and high-intensity statins is to reduce future ischemic events that balance bleeding events and all-cause mortality. The important issues are that the risk factors for ischemic and bleeding events overlap and that efforts to reduce ischemic events using potent or maintenance antithrombotics are closely related to increases in the number of bleeding events. In this study, asymptomatic PAD diagnosed based on an abnormal ABI was also a strong risk factor for the occurrence of ischemic and bleeding events. These findings suggest that various efforts, including appropriate antithrombotic therapy and other CV risk factor control, are needed in these high-risk patients. Recent studies have shown that inflammation or thrombogenicity could be targets for pharmacological treatment of PAD in patients with CAD [30,31]. Translational research may contribute to improving individualized medicine. Prospective studies involving these basic clinical insights are required in the future.
The current study had several limitations. First, the study was a single-center study that used observational cohort data, which may limit the generalizability of the findings. This may have led to an unintended underestimation or overestimation of the ABI, prevalence of clinical events, and hidden confounding variables, which could have resulted in biased outcomes. However, we attempted to minimize any errors in the estimation of incidence by standardizing the inclusion criteria using available resources and by performing a detailed review of all available medical records or telephone interview data. Second, there were significant differences in several factors, including age, presentation of disease entity, hypertension, CKD, and LV systolic function, which may contribute as independent cofounders among the patients with PAD. In the multivariate analysis, adjustments were performed to reduce unexpected bias. Nevertheless, the log-rank test used to compare the Kaplan-Meier survival curves may fail to account for other potential variables, such as lifestyle modifications and medical treatment changes during follow-up, which may have affected the results of the study. Follow-up information of ABI was limited that might affect the long-term clinical outcomes. However, our observational study has strengths in terms of the large-scale study population (n = 4,747) and the evaluation of an abnormal ABI considering the difficulties in performing randomized trials to investigate the influence of this parameter on long-term clinical outcomes.
This study suggests that an abnormal ABI is a risk factor for both ischemic and bleeding events after PCI. In patients undergoing PCI, a diagnostic approach for PAD following intensive management is warranted to improve the long-term clinical outcomes. Our study findings may be helpful in determining the optimal method for secondary prevention after PCI.
1.An abnormal ankle-brachial index (ABI) was an independent risk factor for the primary endpoint (composite of all-cause death, myocardial infarction, stroke, and major bleeding) and major bleeding.
2. Combination of abnormal ABI and reference variables increased predictive value for primary endpoint and major bleeding.
3. Patients with abnormal ABI are at a high risk for both ischemic and bleeding events, and screening and stratifying the risk in patients undergoing percutaneous coronary intervention would be necessary.
Notes
CRedit authorship contributions
Hangyul Kim: data curation, formal analysis, writing - original draft; Seung Do Lee: data curation; Hyo Jin Lee: data curation; Hye Ree Kim: data curation; Kyehwan Kim: data curation; Jin-Sin Koh: data curation; Seok-Jae Hwang: data curation; Jin-Yong Hwang: conceptualization, funding acquisition; Jong-Hwa Ahn: formal analysis, methodology; Yongwhi Park: data curation, project administration; Young-Hoon Jeong: project administration, visualization; Jeong Rang Park: conceptualization, project administration, visualization; Min Gyu Kang: conceptualization, formal analysis, visualization, writing - review & editing
Figure 1.
Flow diagram of the study. Patients who underwent percutaneous coronary intervention (PCI) were eligible for this study. GNUH, Gyeongsang National University Hospital; PAD, peripheral artery disease; ABI, ankle-brachial index.
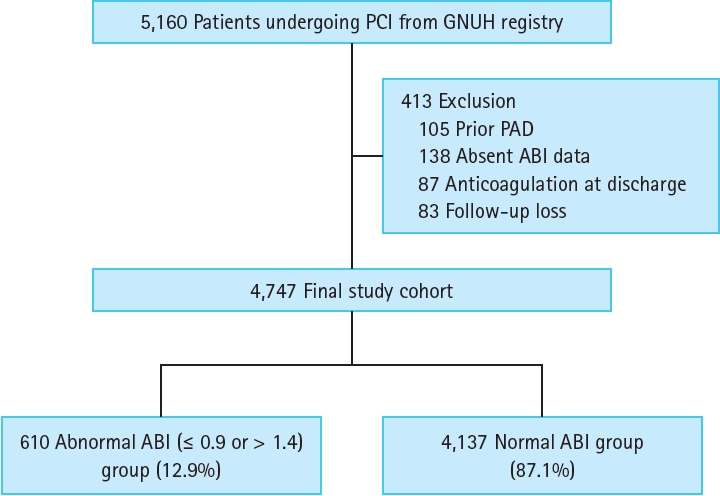
Figure 2.
Five-year cumulative incidence of the clinical outcomes. (A) Primary endpoint: composite of all-cause death, myocardial infarction, stroke, and major bleeding. (B) All-cause death. (C) Myocardial infarction. (D) Stroke. (E) Major bleeding. ABI, ankle-brachial index.
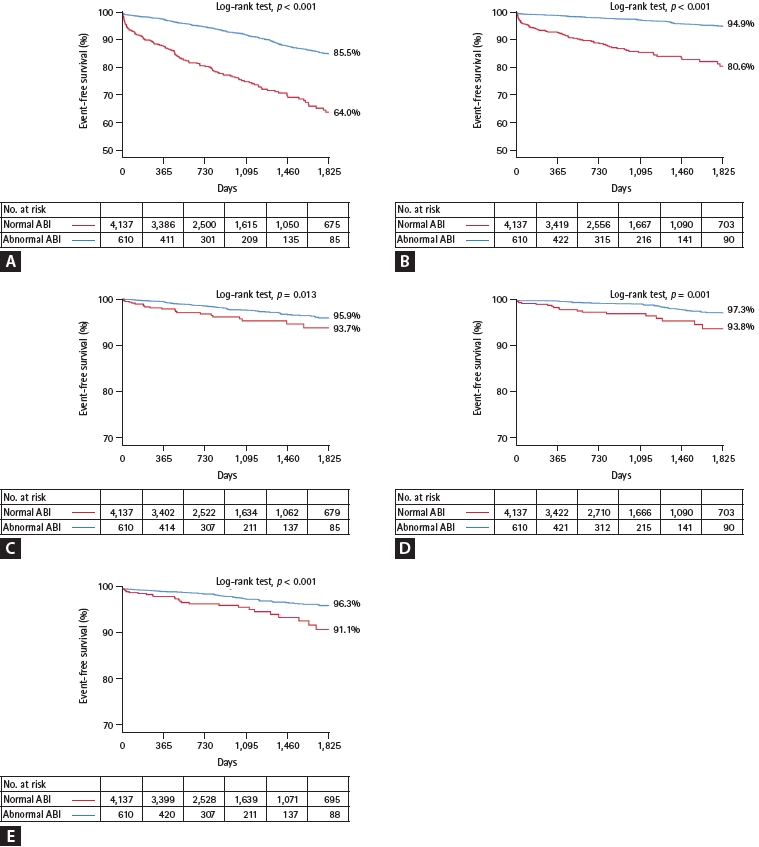
Figure 3.
Predictive discrimination model for primary endpoint and major bleeding. (A) Primary endpoint. (B) Major bleeding. AUC, area under curve; CI, confidence interval; ABI, ankle-brachial index. *Age > 65 years, diabetes, acute coronary syndrome, previous percutaneous coronary intervention, left ventricle ejection fraction < 50%, renal dysfunction (estimated glomerular filtration rate [eGFR] < 60 mL/ min/1.73 m2), and anemia (hemoglobin < 13 g/dL in men, < 12 g/dL in women). †Dyslipidemia, renal dysfunction (eGFR < 60 mL/min/1.73 m2), and anemia (hemoglobin < 13 g/dL in men, < 12 g/dL in women).
Table 1.
Baseline characteristics of patients
Values are presented as mean ± standard deviation or number (%).
ABI, ankle-brachial index; PCI, percutaneous coronary intervention; WBC, white blood cell; eGFR, estimated glomerular filtration rate; NT-pro BNP, N-terminal of the prohormone brain natriuretic peptide; hs-CRP, high sensitivity C-reactive protein; LDL, low density lipoprotein; HDL, high density lipoprotein; LVEF, left ventricle ejection fraction; POBA, plain old balloon angioplasty.
Table 2.
Risk factors for primary endpoint and major bleeding
| Variable |
Univariate analysis |
Univariate analysis |
|||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | ||
| Primary endpoint | |||||||
| Old age (> 65 years) | 1.99 | 1.64–2.42 | < 0.001 | 1.31 | 1.05–1.65 | 0.016 | |
| Acute coronary syndrome | 1.23 | 1.02–1.48 | 0.028 | 1.28 | 1.04–1.57 | 0.019 | |
| Hypertension | 1.34 | 1.11–1.62 | 0.002 | 1.06 | 0.86–1.31 | 0.529 | |
| Diabetes | 1.62 | 1.34–1.96 | < 0.001 | 1.25 | 1.01–1.55 | 0.043 | |
| Dyslipidemia | 1.39 | 1.15–1.68 | 0.001 | 1.17 | 0.96–1.44 | 0.117 | |
| Previous PCI | 1.72 | 1.38–2.15 | < 0.001 | 1.67 | 1.04–2.14 | < 0.001 | |
| Previous stroke | 1.55 | 1.12–2.14 | 0.007 | 1.17 | 0.83–1.66 | 0.350 | |
| Renal dysfunctiona | 2.84 | 2.30–3.80 | < 0.001 | 1.67 | 1.30–2.15 | < 0.001 | |
| Anemiab | 2.17 | 1.75–2.70 | < 0.001 | 1.36 | 1.01–1.67 | 0.045 | |
| Reduced LVEF (< 50%) | 1.80 | 1.47–2.21 | < 0.001 | 1.35 | 1.08–1.68 | 0.007 | |
| Abnormal ABI (≤ 0.9 or > 1.4) | 3.22 | 2.59–4.00 | < 0.001 | 2.21 | 1.74–2.80 | < 0.001 | |
| Major bleeding | |||||||
| Old age (> 65 years) | 2.21 | 1.55–3.15 | < 0.001 | 1.16 | 0.78–1.75 | 0.447 | |
| Acute coronary syndrome | 1.50 | 0.99–2.26 | 0.050 | 1.45 | 0.99–2.12 | 0.055 | |
| Current smoking | 1.51 | 1.04–2.28 | 0.048 | 1.32 | 0.85–2.04 | 0.206 | |
| Dyslipidemia | 1.97 | 1.36–2.85 | < 0.001 | 1.61 | 1.09–2.36 | 0.015 | |
| Renal dysfunctiona | 2.72 | 1.87–3.96 | < 0.001 | 1.64 | 1.07–2.50 | 0.022 | |
| Anemiab | 3.06 | 2.12–4.42 | < 0.001 | 1.82 | 1.22–2.71 | 0.003 | |
| Reduced LVEF (< 50%) | 1.66 | 1.14–2.41 | 0.008 | 1.25 | 0.84–2.41 | 0.256 | |
| Abnormal ABI (≤ 0.9 or > 1.4) | 2.31 | 1.54–3.46 | < 0.001 | 1.61 | 1.03–2.51 | 0.034 | |
REFERENCES
1. Fowkes FG, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK, Criqui MH. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol 2017;14:156–170.



2. Eagle KA, Rihal CS, Foster ED, Mickel MC, Gersh BJ. Long-term survival in patients with coronary artery disease: importance of peripheral vascular disease. The coronary artery surgery study (CASS) investigators. J Am Coll Cardiol 1994;23:1091–1095.

3. Bhatt DL, Peterson ED, Harrington RA, et al. Prior polyvascular disease: risk factor for adverse ischaemic outcomes in acute coronary syndromes. Eur Heart J 2009;30:1195–1202.


4. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2017;135:e726–e779.

5. Bevan GH, White Solaru KT. Evidence-based medical management of peripheral artery disease. Arterioscler Thromb Vasc Biol 2020;40:541–553.


6. Criqui MH, Matsushita K, Aboyans V, et al. Lower extremity peripheral artery disease: contemporary epidemiology, management gaps, and future directions: a scientific statement from the American Heart Association. Circulation 2021;144:e171–e191.



7. Gutierrez JA, Mulder H, Jones WS, et al. Polyvascular disease and risk of major adverse cardiovascular events in peripheral artery disease: a secondary analysis of the Euclid trial. JAMA Netw Open 2018;1:e185239.



8. Jukema JW, Szarek M, Zijlstra LE, et al. Alirocumab in patients with polyvascular disease and recent acute coronary syndrome: ODYSSEY OUTCOMES trial. J Am Coll Cardiol 2019;74:1167–1176.

9. Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319–1330.

10. Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 2018;391:219–229.

11. Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 2012;126:2890–2909.


12. European Stroke Organisation; Tendera M, et al.; Aboyans V. ESC guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the task force on the diagnosis and treatment of peripheral artery diseases of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2851–2906.

13. Lee JY, Lee SW, Lee WS, et al. Prevalence and clinical implications of newly revealed, asymptomatic abnormal ankle-brachial index in patients with significant coronary artery disease. JACC Cardiovasc Interv 2013;6:1303–1313.


14. Ban S, Sakakura K, Jinnouchi H, et al. Association of asymptomatic low ankle-brachial index with long-term clinical outcomes in patients after acute myocardial infarction. J Atheroscler Thromb 2022;29:992–1000.


15. Carter SA. Indirect systolic pressures and pulse waves in arterial occlusive diseases of the lower extremities. Circulation 1968;37:624–637.


16. Suominen V, Uurto I, Saarinen J, Venermo M, Salenius J. PAD as a risk factor for mortality among patients with elevated ABI--a clinical study. Eur J Vasc Endovasc Surg 2010;39:316–322.


17. Garcia-Garcia HM, McFadden EP, Farb A, et al. Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Circulation 2018;137:2635–2650.


18. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011;123:2736–2747.


19. Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006;295:180–189.


20. Hiramori S, Soga Y, Kamioka N, et al. Clinical impact of the ankle-brachial index in patients undergoing successful percutaneous coronary intervention. Circ J 2018;82:1675–1681.


21. Nakahashi T, Tada H, Sakata K, et al. Impact of decreased ankle-brachial index on 30-day bleeding complications and long-term mortality in patients with acute coronary syndrome after percutaneous coronary intervention. J Cardiol 2019;74:116–122.


22. Urban P, Mehran R, Colleran R, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention. Circulation 2019;140:240–261.



23. Cao D, Mehran R, Dangas G, et al. Validation of the Academic Research Consortium high bleeding risk definition in contemporary PCI patients. J Am Coll Cardiol 2020;75:2711–2722.


24. Kim HK, Tantry US, Smith SC Jr, et al. The East Asian paradox: an updated position statement on the challenges to the current antithrombotic strategy in patients with cardiovascular disease. Thromb Haemost 2021;121:422–432.


25. Matsuura Y, Moribayashi K, Kaikita K. Optimal antithrombotic therapy in patients undergoing percutaneous coronary intervention: a focused review on high bleeding risk. J Atheroscler Thromb 2022;29:1409–1420.



26. Sotomi Y, Hikoso S, Nakatani D, et al. Practical assessment of the tradeoff between fatal bleeding and coronary thrombotic risks using the academic research consortium for high bleeding risk criteria. J Atheroscler Thromb 2022;29:1236–1248.



27. Jönelid B, Johnston N, Berglund L, Andrén B, Kragsterman B, Christersson C. Ankle brachial index most important to identify polyvascular disease in patients with non-ST elevation or ST-elevation myocardial infarction. Eur J Intern Med 2016;30:55–60.


28. Ankle Brachial Index Collaboration; Fowkes FG, et al.; Murray GD. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA 2008;300:197–208.



29. Soga Y, Iida O, Takahara M, et al. Two-year life expectancy in patients with critical limb ischemia. JACC Cardiovasc Interv 2014;7:1444–1449.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



