 |
 |
| Korean J Intern Med > Volume 38(2); 2023 > Article |
|
See editorial "Corticosteroids for non-severe COVID-19 infections? Too early to conclude" on page 144.
Abstract
Background/Aims
Corticosteroids (CSs) are frequently used in coronavirus disease 2019 (COVID-19); however, their utility remains controversial in mild to moderate cases. The timing of CSs initiation during the disease course remains unaddressed. The study aims to evaluate the impact of early CSs in non-severe COVID-19.
Methods
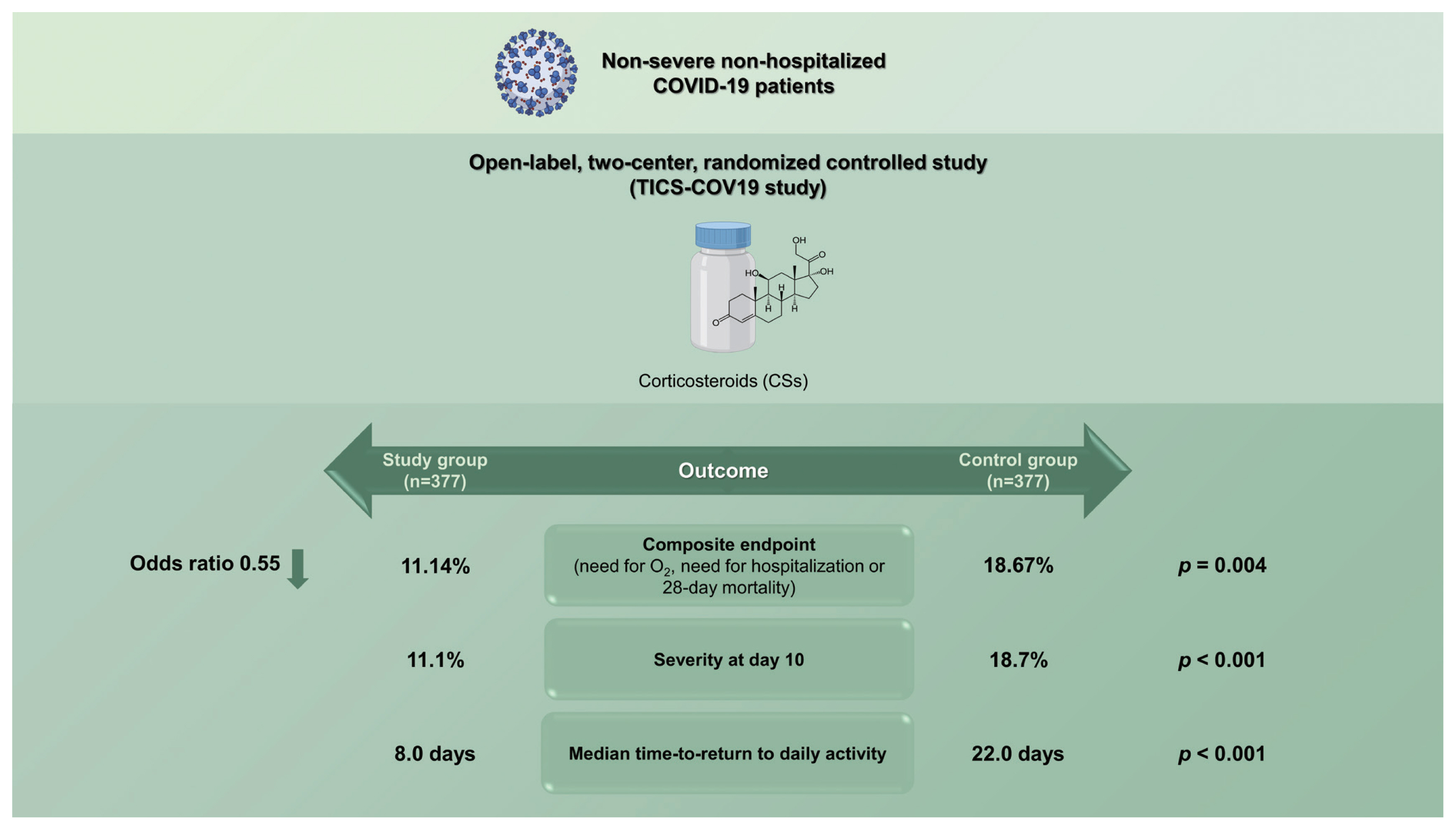
A randomized controlled, open-label study was conducted on 754 COVID-19 patients randomized into a study group (n = 377) in which patients received CSs with COVID-19 protocol and a control group (n = 377) in which patients received COVID-19 protocol only.
Results
Both groups were comparable regarding baseline characteristics, presenting symptoms, and inflammatory markers. The composite endpoint (need for O2, need for hospitalization or 28-day mortality) was significantly (p = 0.004) lower in the CS group 42 (11.14%) versus the control group 70 (18.67%) with odds ratio 0.55 (95% confidence interval [CI], 0.36 to 0.83), absolute risk reduction 7.53% (95% CI, 2.46% to 12.59%) and number needed to treat of 13.29 (95% CI, 7.94 to 40.61). Regarding severity at day 10, only (11.1%) of the study group patients were severe versus (18.7%) of the control group patients (p < 0.001). The median time-to-return to daily activity in the CS group was 8.0 days, while in the control group, it was 22.0 days (p < 0.001).
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused more than 1,150,000 deaths worldwide [1]. Previous research has established that the coronavirus disease 2019 (COVID-19) infection presentation spans from mild self-limiting disease up to severe disease [2], which may manifest by interstitial pneumonia and cytokine storm [3]. Several immunosuppressive medications have been explored to attenuate this hyper-inflammatory reaction associated with COVID-19 due to the lack of a validated treatment [4].
Corticosteroids (CSs) are one of these anti-inflammatory drugs tried to treat COVID-19. Previous research on SARS and Middle East respiratory syndrome (MERS) has established that CSs were associated with delayed viral clearance, with no benefits on clinical outcomes [5]. However, a debate originated when non-randomized studies on COVID-19 demonstrated lower mortality in patients treated with CSs [6,7]. Another prospective meta-analysis supported these preliminary findings [8], incorporating seven randomized clinical trials that showed a survival benefit in critically ill patients with COVID-19 who received CSs [8].
Accordingly, World Health Organization (WHO) recommended systemic CSs to treat patients with severe COVID-19 but non-CSs for those with non-severe COVID-19. Although the findings of seven randomized controlled trials (RCTs) were used to provide recommendations for critical cases, just one RCT, the Randomized Evaluation of COVID-19 Therapy (RECOVERY) study, was used to make recommendations for non-critical situations [9].
Despite Russell et al. [10] reporting that CSs may be beneficial if initiated in the early acute phase of SARS-CoV-2 infection; however, conflicting evidence from the WHO surrounding CSs use in SARS-CoV-2 infection means this evidence is not conclusive. However, on the contrary, Han et al. [11] reported that long-term CSs use might cause atypical infections and delay viral clearance. Tang et al. [12] also recommended caution regarding CSs for COVID-19, not advising their use as a routine treatment.
Of particular concern, CSs have anti-inflammatory, anti-fibrotic, and vaso-constrictive effects. For decades, CSs for acute respiratory distress syndrome (ARDS) have been examined in numerous clinical trials with conflicting results, and most experts do not recommend their use in those settings. On first describing ARDS (1967) by Ashbaugh et al. [13], they declared that ŌĆ£CSs have benefit in treating patients with possibly viral pneumonia.ŌĆØ due to their known diminishing effect on the inflammatory response, the main factor for lung damage and ARDS in SARS-CoV-2 infection. Accordingly, the use of CSs to reduce inflammatory-induced lung injury has been described for patients with severe COVID-19 [3].
Non-severe COVID-19 pneumonia, according to the Center for Disease Control (CDC) and United States National Institute of Health (2020) [14], on the other hand, has received much too little attention. Is it appropriate to allow CSs for those cases? What are the benefits and disadvantages of using CSs for them? Hence, we attempted to evaluate the impact of early treatment with CSs for non-severe COVID-19 on patientsŌĆÖ clinical outcomes and progression versus non-CSs or delayed CSs.
A parallel randomized controlled, open-label was conducted on COVID-19 non-vaccinated patients confirmed to have COVID-19 attending the outpatient clinics of two centers in Cairo, Egypt, from March 2021 to November 2021. COVID-19 was confirmed according to the positive results of SARS-COV-2 nucleotides following the WHO guidelines [15]. The medical research ethical committee (MREC) of the National Research Center (NRC) approved the study with approval number (011022021). The study has clinicaltrials.gov number NCT04530409. Informed consent was obtained from all participants. The nasopharyngeal or throat swab specimens were collected, and the SARS-COV-2 nucleotides were tested using real-time polymerase chain reaction (RT-PCR). Eligibility criteria required individuals who were 18 years or older, male or female, confirmed COVID-19 clinically, radiologically, and by PCR for RNA SARS-CoV-2 of mild or moderate severity with an elevated inflammatory marker (C-reactive protein [CRP], lactate dehydrogenase [LDH], or ferritin). However, patients with immunodeficiency disorders, receiving anti-cancer/immunosuppressive therapy, patients with confirmed bacterial infection, severe cases requiring immediate intensive care unit admission or on chronic steroid therapy or using CSs after progression to severe disease, refusing to participate, or participating in another study were excluded. We assessed the participantsŌĆÖ severity according to the local COVID-19 protocol: Ministry of Health (MOH) protocol version 1.4 November 2020 as follows: Mild cases (mild symptoms & normal computed tomography [CT] imaging & SpO2 > 92%) and moderate cases (positive CT, SpO2 > 92%) [16].
A complete history of COVID-19 symptomatology and clinical examination was performed. Laboratory investigations were carried out in the form of complete blood count with differential and inflammatory markers such as CRP (enzyme-linked immunosorbent assay [ELISA]), ferritin (ELISA), LDH (ELISA), and D-dimer (ELISA). Radiological assessment was carried out by CT chest (80-slice CT machine, Prime Aquilion, Toshiba, Tustin, CA, USA). We compared the clinical outcomes of patients with non-severe COVID-19 treated with and without CSs.
The primary outcome was a composite endpoint of need for O2 at day 10, need for hospitalization at day 10, or 28-day mortality. Secondary outcomes were time-to-return to daily activity, change in severity and inflammatory markers course (D-dimer, CRP, LDH, and ferritin) at day 10.
Return-to-daily activity was defined as returns to work, return to their home works for women, return to their previous activities before catching COVID-19. Besides, any adverse drug reactions of CSs therapy were recorded during the study period. As per the study protocol, it was not planned to follow-up on the long-term outcomes of CSs as a low to moderate dose (15 to 30 mg) of methylprednisolone was used for a short period of less than 3 months. Thus, it was not a long-term use. All patients were followed up on day 3, day 10, and every week for 45 days or until recovery or hospitalization, or death. Telephone calls were made whenever patients missed a visit.
The study was open-label. Block randomization was conducted using computer-generated random numbers with block size four and an allocation ratio of 1:1. All participants were subjected to management using the local COVID-19 protocol: MOH protocol version 1.4 November 2020 according to the severity (antipyretics, antivirals, antibiotics, and anticoagulants if required). The enrolled patients were randomized into two groups. The CSs group (the active group) was managed according to the standard protocol in addition to CSs once diagnosed (methylprednisolone 30 mg daily for 1 week in moderate cases, then reduced gradually over 2 weeks). In mild cases, we started 15 mg only.
The control group was subjected only to the standard COVID-19 protocol. Control subjects who worsened (needed O2 supplementation or needed hospitalization were managed according to the standard COVID-19 protocol). Thus, they received CSs after their deterioration (delayed CSs). Every visit patient comes, he/she is asked to bring his/her medications with him/her to follow-up on the utilization of CSs.
This study used all patients fulfilling the eligibility criteria as a sampling technique. As there was no published data about the use of CSs in mild to moderate cases of COVID-19 at the time of the design of this study, a pilot study of 30 cases was carried out to help determine the sample size for the current study. This pilot study found that 27% of the non-CS group have the composite endpoint versus 7% of the CS group.
Thus, with significance level ╬▒ = 0.05 and 95% power (╬▓ = 0.05), 122 cases in each arm were needed. Assuming an equal sample size in each group, the total sample needed was 122 ├Ś 2 = 244, with an expected dropout rate of 20%, so the sample size will be 300 cases. We increased the sample size to more than 700 cases to allow for subgroup analyses.
The statistical analysis for efficacy and safety was made on the intent-to-treat population. All statistical tests were done using a significance level of 95%. A value of p < 0.05 was considered statistically significant. SPSS software version 25.0 (IBM Co., Armonk, NY, USA) was used for the statistical analyses. Data was presented as mean ┬▒ standard deviation for continuous variables, median (interquartile range [IQR]) for ordinal and non-parametric data, and frequency & percentage for categorical variables. Comparisons were made using the Pearson chi-square or Phi test for categorical variables, the unpaired StudentŌĆÖs t test for normal continuous variables, and the Mann-Whitney test for non-parametric data. The statistical analysis for the primary outcome measure was made by odds ratio (OR) absolute risk reduction (ARR) and number needed to treat (NNT), all with a 95% confidence interval (CI).
A total of 1,049 patients were asked to participate in this study. Thirty-six patients refused to participate, 167 patients were severe cases, and 87 patients had within normal levels inflammatory markers at presentation; leaving 754 patients for randomization with 377 assigned to each group as follows: study group (steroid group, n = 377), which included patients who received CSs (methylprednisolone 30 mg/day) in addition to the standard COVID-19 treatment protocol. The control group (n = 377) only included patients who received the COVID-19 treatment protocol. Two patients from the control group were excluded after randomization because of loss of contact before the second visit (Fig. 1).
The study and control groups were comparable regarding gender, age, body mass index, smoking, and comorbid conditions (diabetes mellitus [DM] and hypertension), as shown in Table 1. Besides, no statistically significant (p = 0.718) difference was detected between both groups regarding the severity of COVID-19 (Table 1). The majority of cases, 67.1% and 65.9% of the CS and control groups, respectively, were moderate cases.
Both groups were comparable regarding the presenting symptoms (p > 0.05). Cough is the most frequent symptom in the CS group (69.5%) versus the control group (72.5%) (p = 0.359). Leucopenia was observed in 152 (40.3%) and 151 (40.3%) of the CS group and the control group, respectively (p = 0.988). In addition, neutropenia was seen in 170 (45.1%) and 166 (44.3%) of the CS group and the control group, respectively (p = 0.82). Lymphopenia was seen in 154 (40.8%) and 154 (41.1%) of the CS group and the control group, respectively (p = 0.952).
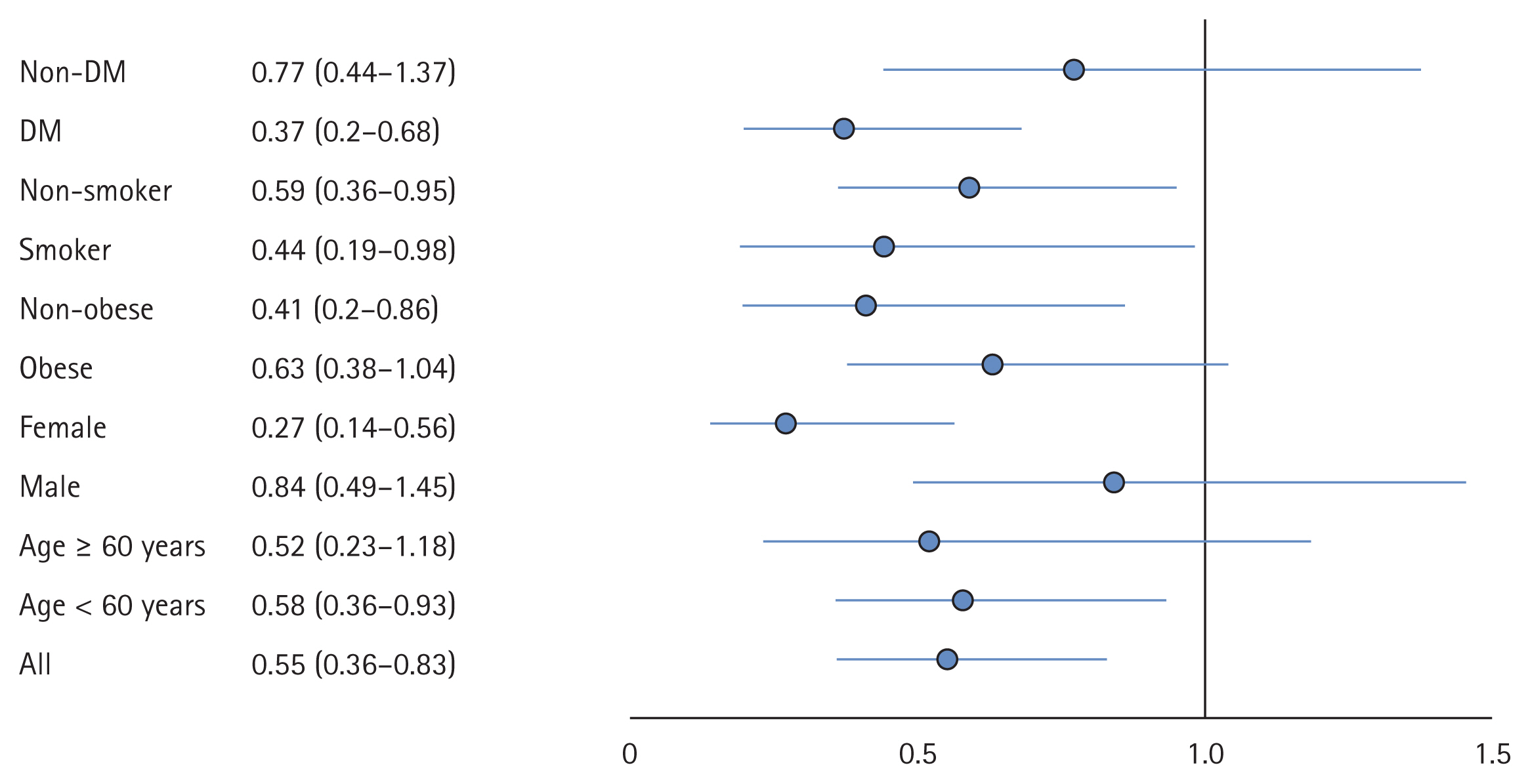
The composite endpoint (need for O2, need for hospitalization or 28-day mortality) was significantly (p = 0.004) lower in the CS group 42 (11.14%) versus the control group 70 (18.67%) with OR 0.55 (95% CI, 0.36 to 0.83), ARR 7.53% (95% CI, 2.46% to 12.59%), and NNT of 13.29 (95% CI, 7.94 to 40.61). Further, need for O2 supplementation was significantly (p = 0.004) lower in the CS group 42 (11.2%) than the control group 70 (18.67%) with OR 0.55 (95% CI, 0.36 to 0.83), ARR 7.53% (95% CI, 2.46% to 12.59%), and NNT of 13.29 (95% CI, 7.94 to 40.61). Also, need for hospitalization was significantly (p = 0.001) less in the CS group 24 (6.37%) than the control group 51 (13.6%) with OR 0.43 (95% CI, 0.26 to 0.72), ARR 7.23% (95% CI, 2.98% to 11.49%), and NNT of 1,382 (95% CI, 8.70 to 33.58). The 28-day mortality was significantly (p = 0.043) less in the CS group 3 (0.80%) than the control group 11 (2.93%) with OR 0.27 (95% CI, 0.07 to 0.96), ARR 2.13% (95% CI, 0.21% to 4.07%), and NNT of 46.78 (95% CI, 24.59 to 479.50).
Analyses of the primary outcome in different subgroups, namely, age group (60 years), gender, obesity, smoking, DM, and smoking, were carried out (Fig. 2). The benefit of CSs was more evident in the age group less than 60 years OR 0.58 (95% CI, 0.36 to 0.93; p = 0.025), the female gender OR 0.27 (95% CI, 0.14 to 0.56; p < 0.001), the non-obese OR 0.41 (95% CI, 0.2 to 0.86; p = 0.018), and the DM group OR 0.37 (95% CI, 0.2 to 0.68; p = 0.002).
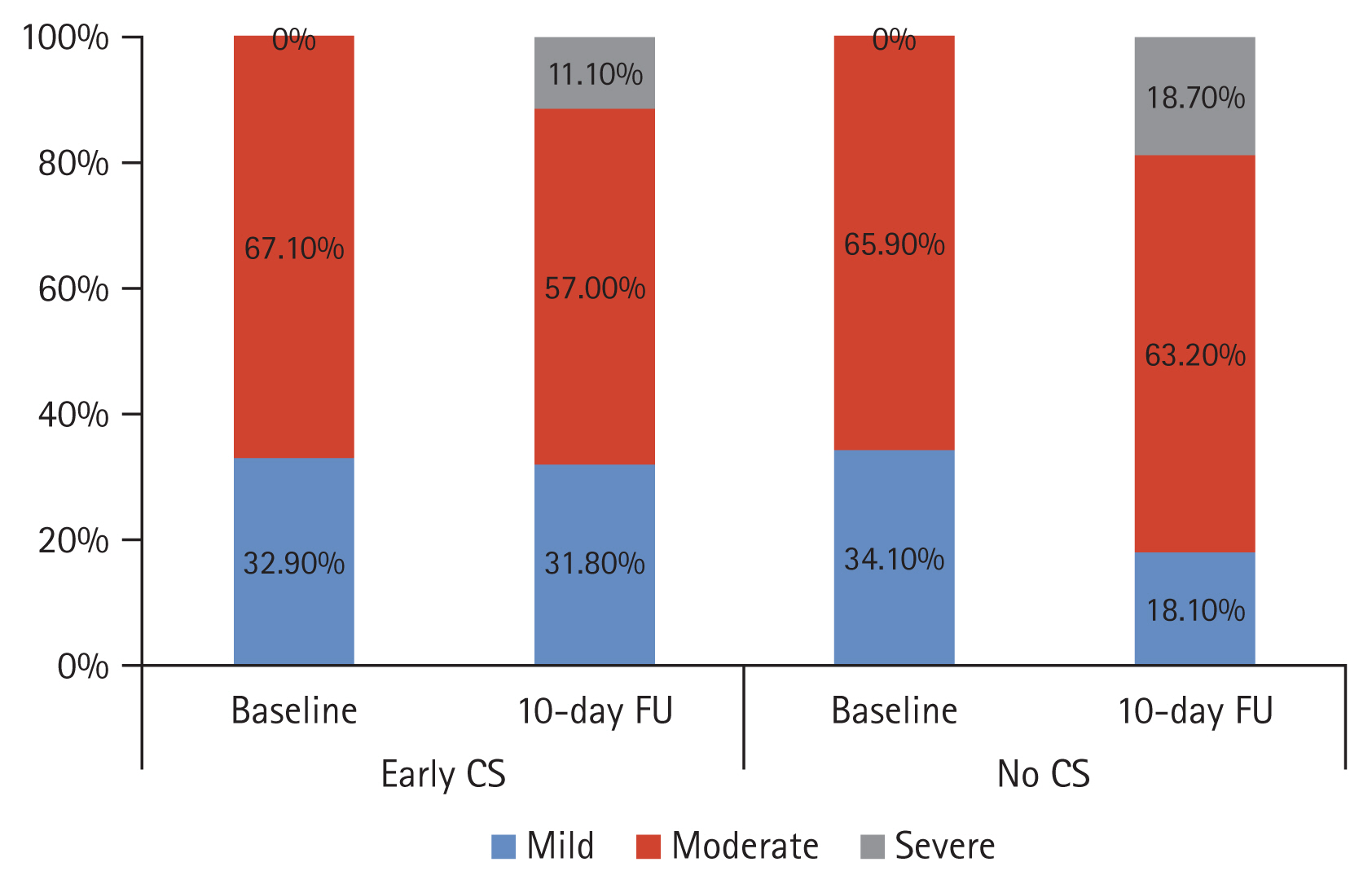
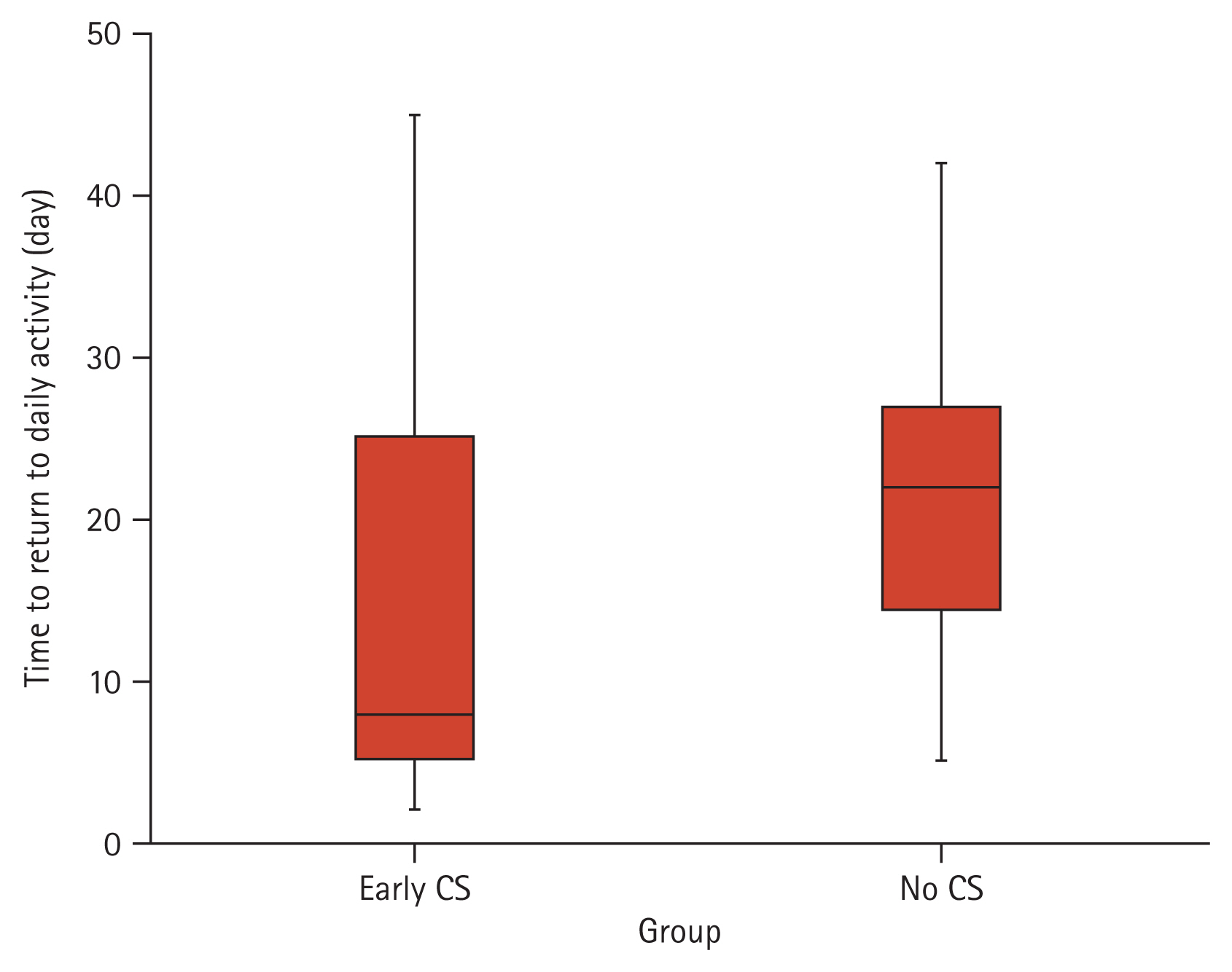
A closer inspection of Fig. 3 shows that severity at day-10 follow-up differed between groups (p < 0.001). Only 42 (11.1%) CS group patients were severe versus 70 (18.7%) of the control group patients. The median time-to-return to daily activity in the CS group was 8.0 days (IQR, 5.0 to 25.0), while in the control group, it was 22.0 days (IQR, 14.2 to 27.0; p < 0.001), as shown in Fig. 4. At presentation, both groups were comparable regarding the inflammatory markers (CRP, ferritin, LDH) as well as the D-dimer (p > 0.05). However, it can be seen from the data in Table 2 significant differences (p < 0.05) at day-10 evaluation, with a more significant reduction in the CS group compared to the control group.
In the CS group, mild elevation of blood pressure was seen in 10 (2.7%) of patients, mild elevation of blood glucose level 8 (2.1%), numbness 24 (6.4%), and epigastric discomfort 6 (1.6%). These adverse events did not necessitate the discontinuation of CSs.
Concerns against using CSs in COVID-19 are the potential negative impact of CSs on viral clearance and the potential side effects, particularly secondary infections. However, in severe/critical cases, the benefits of CSs administration outweigh the hazards, mainly because some COVID-19 patients have a biphasic illness progression with a benign presentation followed by subsequent respiratory deterioration [17].
Theoretically, early CSs therapy might reduce the inflammatory response and prevent the progression of COVID-19. However, data about the efficacy, appropriate initiation timing, and CS dose of CSs in COVID-19 are much less known. CSs boast immunosuppressive and anti-inflammatory properties by inhibiting the transcription of cytokines like interleukin 6, which remains a hallmark of the unusual immune response characteristic of a COVID-19 infection. Accordingly, CSs can effectively decrease mortality rate, oxygen supplementation, and the length of mean hospital stays in those cases infected with severe COVID-19 [18].
Some of the medical literature has further suggested that administering low-dose methylprednisolone for a short period in the early phases of COVID-19 can prevent the progression in cases with mild or moderate severity. Therefore, this study seeks to obtain data that will help address these research gaps regarding the impact of early treatment with low-dose, short-term CSs therapy on the clinical outcomes in adults with non-severe COVID-19.
In the current study, methylprednisolone was used, not dexamethasone, because it achieves higher lung tissue-to-plasma ratios in animal models than dexamethasone and thus has higher lung penetration [19,20]. Also, the effectiveness of methylprednisolone in treating SARS disease has been evidenced [21]. Moreover, a triple-blinded RCT investigating which CS, methylprednisolone, or dexamethasone is superior in treating hospitalized COVID-19 patients. The study found that methylprednisolone demonstrated better results than dexamethasone [22]. The current study was conducted on 754 patients randomized into two comparable groups the study group (CS group) and the control group. The need for O2 supplementation was significantly (p = 0.004) lower in the CS group 42 (11.1%) compared to the control group 70 (18.7%). Besides, need for hospitalization was significantly (p = 0.001) less in the CS group 24 (6.4%) than the control group 51 (13.6%). Another important finding is the significant reduction of the 28-day mortality (p = 0.03) in the CS group 3 (0.8%) compared to the control group 11 (2.9%), with OR 0.27 (95% CI, 0.08 to 0.98). These accords with earlier observations in the RECOVERY trial which showed that in patients hospitalized with severe COVID-19 disease, the uptake of CSs decreased the mortality rate when compared to that seen in the non-CSs group (22.9% and 25.7%, respectively; p < 0.001) [8].
The current guidelines for treatment have vouched for the uptake of CSs only in severe disease. Nothing has been said about using CSs early in the illness course to stop a mild or moderate COVID-19 infection from progressing. Guidelines recommendation against using CSs in mild to moderate non-hospitalized cases was based upon the fact that ŌĆ£There are no data to support the use of systemic CSs in non-hospitalized patients with COVID-19ŌĆØ [23]. Also, the WHO recommendation against using CS in mild to moderate cases was based upon subgroup analysis of the RECOVERY trial. This subgroup analysis was not planned during the design of the study. Therefore, it is non-conclusive [13]. However, our randomized controlled study is planned to answer this question. Thus, it may help close the gap and present a piece of evidence.
Spagnuolo et al. [24] showed that CSs treatment has no impact on delayed viral clearance in patients with moderate or severe COVID-19. The authors observed that delayed viral clearance was associated with older age as well as longer duration of symptoms before hospitalization and respiratory impairment and lymphopenia at admission [24]. However, Spagnuolo et al. [24] observed a longer hospitalization length in CSs users than non-users. In our study, we just depend on clinical data as well as inflammatory markers, which improved in the CSs group compared to the control group.
In the current study, the benefits of CSs (need for O2 supplementation, hospitalization, and 28-mortality) were more evident in the CS group than in the control group in the age group < 60 years, the female gender, the non-obese, and the DM group (p < 0.05). These were consistent with Almas et al. [25], who conducted a study on 25 patients with non-severe COVID-19 infection who received a low-dose, short-course CS for 7 days. The mortality rate was significantly lower in the CSs group compared with the non-CSs group (8.3% and 61.5%, respectively; p = 0.005), and the prevalence of ARDS was (16.7% and 84.6%, respectively; p = 0.002). The incidence of developing secondary complications within the CSs group was also markedly lower than that in the non-CSs group [25].
In the current study, the benefits of CSs were more in females than males. Different theories have been suggested to explain that finding. Perhaps estrogens have a protective role. Also, genetics and epigenetics factors may account for the disparity in mortality among men and women with COVID-19 more than sex steroid hormones [26]. However, this finding of the current RCT needs more investigation in a pre-planned study with the objective of finding the gender difference of CSs effects in COVID-19 as it is beyond the objective of our study.
Also, the study showed that the benefits of CSs were more prominent in DM cases. In the era of the COVID-19 pandemic, more consideration for managing COVID-19 in DM cases has been made. For this particular case, the management of DM was stricter, as published in one research study in 2020 [27]. Also, a strict follow-up, teaching them to self-check their glucose level and manage accordingly. Open communication via text messages and phone calls to help manage them on time. We have much fear about them all.
On the contrary, some studies have warned against using CSs in COVID-19 early in the disease course due to their potential immunosuppressive properties, as evidenced by their use in previous pandemics. In MERS-CoV, there was delayed viral clearance in those who received CSs. Similarly, in SARS-CoV-1, viral clearance was significantly lower in the CSs group [28,29]. Therefore, despite that, the efficacy of CSs in the management of non-severe COVID-19 infection is self-evident in our study; viral clearance should be taken into consideration, which is one limitation of the current research as we did not measure it.
Almost all other studies were conducted on severe COVID-19. To our knowledge, scanty studies have been conducted on non-severe COVID-19. Dupuis et al. [30] conducted an observational study on critically ill 303 patients with COVID-19 and recorded that early CSs treatment was associated with a lower mortality rate (p = 0.03). However, CSs were associated with an increased risk of death in patients younger than 60 years without inflammation on admission (p = 0.04) [30]. In addition, Tang et al. [31] investigated the efficacy and safety of CSs given to hospitalized patients with COVID-19 pneumonia. They detected no difference in the incidence of clinical deterioration between the CSs group and the control group (4.8% vs. 4.8%, p = 1.000). The two groups were comparable concerning secondary outcomes.
Interestingly in the current study, the severity at day-10 follow-up was different between both groups (p < 0.001). Only 42 (11.1%) CS group patients were severe versus 70 (18.7%) of the control group patients. The median time-to-return to daily activity in the CS group was 8.0 days (IQR, 5.0 to 25.0), while in the non-CSs group, it was 22.0 days (IQR, 14.2 to 27; p < 0.001). Despite comparable inflammatory markers of both groups at the start of the study, we noticed significant differences (p < 0.05) at the day-10 evaluation, with a more significant reduction in the CS group than the control group.
In accordance with the present results, the previous study by Almas et al. [25] has demonstrated that CRP values in the CSs group were significantly lower at seven days when compared to the non-CSs group (14.53 and 44.43 mg/L, respectively; p < 0.001). Moreover, there was a marked reduction in the length of hospital stay within the CSs group compared to the control group (14.23 and 20.16 days, respectively; p < 0.001) [25].
However, Ling et al. [32] observed that the duration of SARS-CoV-2 RNA in the CS group was longer than that in the control group (15 days vs. 8.0 days, p = 0.013). Besides, Ma et al. [33] proposed that SARS-CoV-2 RNA clearance would be delayed because of the immunosuppressive effect of the higher dose of CSs. Li et al. [17], who studied ten cohort studies and one RCT found that CSs use in subjects with SARS-CoV-2, SARS-CoV, and MERS-CoV infections delayed virus clearing. A retrospective study by Yuan et al. [34] also found that the CS group had a longer duration of SARS-CoV-2 shedding (20.3 days vs. 19.4 days, p = 0.669).
In addition, Zha et al. [35] found a prolonged duration of symptoms (median 8 days vs. 6.5 days) in the CS group compared to the control group. Nevertheless, Yuan et al. [34] found no statistically significant differences in the duration of fever (9.5 days vs. 10.2 days, p = 0.28) between patients who received and those who did not receive CSs treatment. Xu et al. [36] reported a case with COVID-19 treated with CSs since day 8 of the disease course but had worsened disease course, and developed respiratory failure and died on day 14. This may be attributed to the delayed initiation of CSs.
One meta-analysis from 15 studies found that CSs treatment was associated with higher mortality (p = 0.019), longer hospital stays (p < 0.001), and a higher rate of bacterial infection (p < 0.001) in patients with COVID-19 [37]. However, all included studies in the meta-analysis were retrospective cohort studies, not RCTs.
Yuan et al. [34] also found that the CS group had more patients with non-severe COVID-19 pneumonia who developed severe disease (11.4% vs. 2.9%, p = 0.353) than the control group. However, the studyŌĆÖs retrospective nature with a small sample size of 132 patients was based on propensity score analysis.
Li et al. [17] studied 475 patients with non-severe COVID-19 pneumonia, of which 55 received early CSs therapy; 420 patients served as control. More cases developed severe disease (12.7% vs. 1.8%, p = 0.028) in the CS group compared to the control group. Nevertheless, no significant difference between groups in mortality (1.8% vs. 0%, p = 0.315) [17]. However, this study was a retrospective cohort study and was not randomized which might affect the results. Lastly, Yu et al. [38] detected that inhaled CSs improved time to recovery, with a reduction of hospital admissions or deaths.
These conflicting and contradictory results in the previous studies reported may be attributed to many factors as delayed initiation of CSs therapy in some studies, also the dose of CSs, mode of administration and its duration in addition to the selectivity of patients and the different sample size between compared groups as well as the presence of other confounding factors that may affect results as age.
The pathophysiology behind the lung damage in COVID-19 is linked to an uncontrolled pro-inflammatory cytokine response [39]. CSs anti-inflammatory effect plays a role in mitigating this hyper-inflammatory reaction. Hence, in our current work, we started CSs therapy once high levels of inflammatory markers confirmed hyper-inflammatory status. The current study had some limitations as the lack of a placebo and the detection of viral clearance. We depend in our study on the history and clinical improvement as well as the regression of inflammatory markers to their normal levels.
In conclusion, CSs regimen must be performed delicately, respecting the time of initiation and dose concerning its immunosuppressive effects to avoid any superimposed infections. Therefore, a short-course low-dose CSs might confer a therapeutic advantage by curbing the progression of the non-severe disease and avoiding potentially life-threatening complications. This study has identified that using early, low-dose, short-course CSs effectively reduced the mortality in non-severe COVID-19 and the time-to-return to daily activities. Furthermore, CSs were associated with significantly ameliorating the patientsŌĆÖ clinical outcomes, as evidenced by significantly reduced severity and levels of inflammatory markers. Improved outcome in mild/moderate COVID-19 was better in the early use of CSs in those aged less than 60 years.
1. The lung damage in coronavirus disease 2019 (COVID-19) is linked to an uncontrolled pro-inflammatory cytokine response. Corticosteroids (CSs) anti-inflammatory effect plays a role in mitigating this reaction. Hence, CSs therapy was started in the current work once high inflammatory markers had confirmed the hyperinflammatory status.
2. A short course of low-dose CSs confers a therapeutic advantage by curbing the progression of the non-severe disease and avoiding potentially life-threatening complications.
3. This study has identified that using CSs early effectively reduced the mortality in non-severe COVID-19 and the time-to-return to daily activities and was associated with significantly improved clinical outcomes.
Figure┬Ā1
CONSORT diagram. COVID-19, coronavirus disease 2019; CS, corticosteroid; ITT, intent-to-treat; PP, per protocol.
Figure┬Ā3
Change in severity of coronavirus disease 2019 (COVID-19) in both studied groups at day-10 follow-up (HU) visit. CS, corticosteroid.
Table┬Ā1
Demographic and clinical characteristics of both studied groups
Table┬Ā2
Inflammatory markers and D-dimer in both groups: baseline and 10-day evaluation
REFERENCES
1. Coronavirus Resource Center Johns Hopkins University & Medicine. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) [Internet] Baltimore (MD): Johns Hopkins University & Medicine, 2022. [cited 2022 Nov 21]. Available from: https://coronavirus.jhu.edu/map.html
. 2020.
2. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061ŌĆō1069.



3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497ŌĆō506.



4. Salvi R, Patankar P. Emerging pharmacotherapies for COVID-19. Biomed Pharmacother 2020;128:110267.



5. Li H, Chen C, Hu F, et al. Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis. Leukemia 2020;34:1503ŌĆō1511.




6. Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 2020;395:683ŌĆō684.



7. Fadel R, Morrison AR, Vahia A, et al. Early short-course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis 2020;71:2114ŌĆō2120.



8. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne JA, et al.; Murthy S. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020;324:1330ŌĆō1341.

9. Angus DC, Derde L, Al-Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA 2020;324:1317ŌĆō1329.



10. Russell B, Moss C, Rigg A, Van Hemelrijck M. COVID-19 and treatment with NSAIDs and corticosteroids: should we be limiting their use in the clinical setting? Ecancermedicalscience 2020;14:1023.



11. Han Y, Jiang M, Xia D, et al. COVID-19 in a patient with long-term use of glucocorticoids: a study of a familial cluster. Clin Immunol 2020;214:108413.



12. Tang C, Wang Y, Lv H, Guan Z, Gu J. Caution against corticosteroid-based COVID-19 treatment. Lancet 2020;395:1759ŌĆō1760.



13. Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet 1967;2:319ŌĆō323.


14. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): cases in U.S. [Internet] Atlanta (GA): CDC, 2020. [cited 2022 Nov 22]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/index.html
.
15. World Health Organization. Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance [Internet] Geneva (CH): WHO, 2020. [cited 2022 Nov 22]. Available from: https://apps.who.int/iris/handle/10665/330893
.
16. Masoud HH, Elassal G, Zaky S, et al. Management protocol for COVID-19 patients: version 1.4 [Internet] Cairo (EG): Ministry of Health and Population, 2020. [cited 2022 Nov 22]. Available from: http://www.mohp.gov.eg/JobsDetails.aspx?-job_id=3061
.
17. Li Q, Li W, Jin Y, et al. Efficacy evaluation of early, low-dose, short-term corticosteroids in adults hospitalized with non-severe COVID-19 pneumonia: a retrospective cohort study. Infect Dis Ther 2020;9:823ŌĆō836.




18. Chatterjee K, Wu CP, Bhardwaj A, Siuba M. Steroids in COVID-19: an overview. Cleve Clin J Med 2020;Aug. 20. [Epub]. https://doi.org/10.3949/ccjm.87a.ccc059
.


19. Annane D, Pastores SM, Arlt W, et al. Critical illness-related corticosteroid insufficiency (CIRCI): a narrative review from a Multispecialty Task Force of the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM). Intensive Care Med 2017;43:1781ŌĆō1792.



20. Meduri GU, Siemieniuk RA, Ness RA, Seyler SJ. Prolonged low-dose methylprednisolone treatment is highly effective in reducing duration of mechanical ventilation and mortality in patients with ARDS. J Intensive Care 2018;6:53.




21. Papamanoli A, Yoo J, Grewal P, et al. High-dose methylprednisolone in nonintubated patients with severe COVID-19 pneumonia. Eur J Clin Invest 2021;51:e13458.




22. Ranjbar K, Moghadami M, Mirahmadizadeh A, et al. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect Dis 2021;21:337.




23. COVID-19 Treatment Guidelines. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines [Internet] Bethesda (MD): National Institutes of Health, 2022. [cited 2022 Nov 22]. Available from: https://www.covid19treatmentguidelines.nih.gov
.
24. Spagnuolo V, Guffanti M, Galli L, et al. Viral clearance after early corticosteroid treatment in patients with moderate or severe covid-19. Sci Rep 2020;10:21291.



25. Almas T, Ehtesham M, Khan AW, et al. Safety and efficacy of low-dose corticosteroids in patients with non-severe coronavirus disease 2019: a retrospective cohort study. Cureus 2021;13:e12544.



26. Traish AM. Sex steroids and COVID-19 mortality in women. Trends Endocrinol Metab 2021;32:533ŌĆō536.



27. Alshaikh A, Alsifri S, Alhozali A, et al. Saudi Scientific Diabetes Society position statement: management of diabetes mellitus in the pandemic of COVID-19. Int J Clin Med 2020;11:199ŌĆō206.


28. Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med 2018;197:757ŌĆō767.


29. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clin Virol 2004;31:304ŌĆō309.



30. Dupuis C, de Montmollin E, Buetti N, et al. Impact of early corticosteroids on 60-day mortality in critically ill patients with COVID-19: a multicenter cohort study of the OUTCOMEREA network. PLoS One 2021;16:e0255644.



31. Tang X, Feng YM, Ni JX, et al. Early use of corticosteroid may prolong SARS-CoV-2 shedding in non-intensive care unit patients with COVID-19 pneumonia: a multicenter, single-blind, randomized control trial. Respiration 2021;100:116ŌĆō126.




32. Ling Y, Xu SB, Lin YX, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020;133:1039ŌĆō1043.



33. Ma SQ, Zhang J, Wang YS, et al. Glucocorticoid therapy delays the clearance of SARS-CoV-2 RNA in an asymptomatic COVID-19 patient. J Med Virol 2020;92:2396ŌĆō2397.




34. Yuan M, Xu X, Xia D, et al. Effects of corticosteroid treatment for non-severe COVID-19 pneumonia: a propensity score-based analysis. Shock 2020;54:638ŌĆō643.


35. Zha L, Li S, Pan L, et al. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19). Med J Aust 2020;212:416ŌĆō420.


36. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420ŌĆō422.



37. Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect 2020;81:e13ŌĆōe20.



-
METRICS

- Related articles
-
Duration of corticosteroid treatment in hospitalized COVID-19 patients, less is more2024 March;39(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


