 |
 |
| Korean J Intern Med > Volume 37(4); 2022 > Article |
|
Abstract
Background/Aims
Recurrent acute myocardial infarction (AMI) is an adverse cardiac event in patients with a first AMI. The predictors of recurrent AMI after the first AMI in patients who underwent successful percutaneous coronary intervention (PCI) have not been elucidated.
Methods
We analyzed the data collected from 9,869 patients (63.2 ± 12.4 years, men:women = 7,446:2,423) who were enrolled in the Korea Acute Myocardial Infarction Registry-National Institute of Health between November 2011 and October 2015, had suffered their first AMI and had received successful PCI during the index hospitalization. Multivariable logistic regression analysis was performed to identify the independent predictors of recurrent AMI following the first AMI.
Cardiovascular disease is one of the major causes of death globally and in the Republic of Korea [1,2]. Therefore, many trials are being conducted with the aim of decreasing mortality and improving the outcomes after acute myocardial infarction (AMI).
Among the complications of AMI, recurrent AMI is a very critical condition and can be fatal. According to a previous study, the mortality risk of recurrent AMI is approximately 8% [3]. Furthermore, recurrent AMI impairs cardiac function, which leads to heart failure and its symptoms that adversely affect patients’ quality of life. Thus, identifying patients who are at risk for recurrent AMI after successful percutaneous coronary intervention (PCI) is effective in implementing active surveillance and strategies to manage modifiable risk factors in these patients. These efforts are expected to improve health outcomes, decrease mortality rate, preserve patients’ cardiac function, and enhance their quality of life. However, only a few studies have focused on the risk factors for recurrent AMI. Herein, we aimed to elucidate the risk factors for recurrent AMI despite successful PCI by comparing the characteristics of patients who did and did not suffer from recurrent AMI. We investigated the independent predictors for recurrent AMI following the first AMI despite successful PCI using the Korea Acute Myocardial Infarction Registry (KAMIR)-National Institute of Health (NIH) data.
We used data from the KAMIR-NIH registry [2]. The KAMIR-NIH registry was approved by the ethics committee at each participating center (Chonnam National University Hospital; CNUH-2011-172), this study was approved by the Institutional Review Board of Chonnam National University Hospital (IRB No : CNUH-2022-058) and written informed consent was obtained from all patients. Between November 2011 and October 2015, 13,103 patients were enrolled in the KAMIR-NIH (Fig. 1). We excluded 1,029 cases with a history of previous myocardial infarction (MI); thus, 12,074 patients who had experienced a first AMI were included. Among them, PCI was not performed in 1,168 cases, the PCI was suboptimal in 94 cases, or failed in 53 cases. Therefore, 10,759 patients underwent successful PCI, of whom 890 were lost to follow-up. Finally, 9,869 patients were included in this study, and AMI recurred in 359 cases.
AMI was defined as evidence of myocardial injury, with an elevation of cardiac troponin levels where at least one value was above the 99th percentile of the upper reference limit, and with the evidence of necrosis consistent with myocardial ischemia identified in a clinical setting. Clinical findings consistent with myocardial ischemia included at least one of the following: (1) symptoms such as chest pain or discomfort; (2) electrocardiogram abnormalities (ST segment elevation at the J point in more than two continuous leads [over 0.2 mV elevation in V2–V3, 0.1 mV in other leads], ST segment changes except for elevation [downslope or horizontal ST segment depression over 0.05 mV, T wave inversion in more than two continuous leads], or newly detected left bundle branch block); and (3) imaging studies, such as echocardiography, suggestive of MI. In this study, recurrent AMI was defined as any spontaneous AMI after the first AMI. Periprocedural MI was not counted in KAMIR-NIH [4]. In the KAMIR-NIH database, a successful PCI was defined as post-thrombolysis in myocardial infarction (TIMI) flow ≥ 2 and residual stenosis < 50%. Renal dysfunction was defined as creatinine clearance > 1.3 mg/dL according to the Global Registry of Acute Coronary Events score [5]. The target value for dyslipidemia was low-density lipoprotein cholesterol (LDL-C) below 70 mg/dL and ≥ 50% reduction from baseline. Obesity was defined as a body mass index > 25 kg/m2 according to the World Health Organization recommendation for Asian populations [6]. The total ischemic time was defined as the time from symptom onset to balloon time. Typical chest pain was defined as substernal chest discomfort of characteristic quality and duration, provoked by exertion or emotional stress and relieved by rest or nitroglycerin. Atypical chest pain was defined as chest pain not consistent with all the characteristics of typical chest pain. Major adverse cardiovascular events (MACE) were defined as the composite of total death, MI, stroke, and revascularization, including PCI and coronary artery bypass graft. The primary endpoint of the study was the first recurrence of AMI after successful PCI for the first AMI.
Continuous variables are presented as mean ± standard and were compared using the Student’s t test or Kruskal-Wallis test. Categorical variables are expressed as number of cases (percentages) and were compared using the chi-square test or Fisher exact test. Each variables about baseline characteristics and clinical findings were chosen based on previous studies [7–12]. We analyzed the relationship between each factor and the recurrence of AMI using univariable logistic regression. Considering the correlation between factors, if the p value was < 0.1 in the univariable logistic regression, we included the factors in the multivariable logistic regression. Finally, we performed the multivariable logistic regression with the selected factors using the backward elimination procedure and likelihood ratio. In logistic regressions, odds ratio and 95% confidence intervals were calculated. Statistical significance was defined as p < 0.05 in the multivariable logistic regression. Additionally, we used Kaplan-Meier curve analysis with log-rank test to investigate the association of recurrent AMI with the difference of MACE incidence after 1 year between patients with and without AMI recurrence.
All statistical analyses were performed using IBM SPSS Statistics version 26 (IBM Co., Armonk, NY, USA).
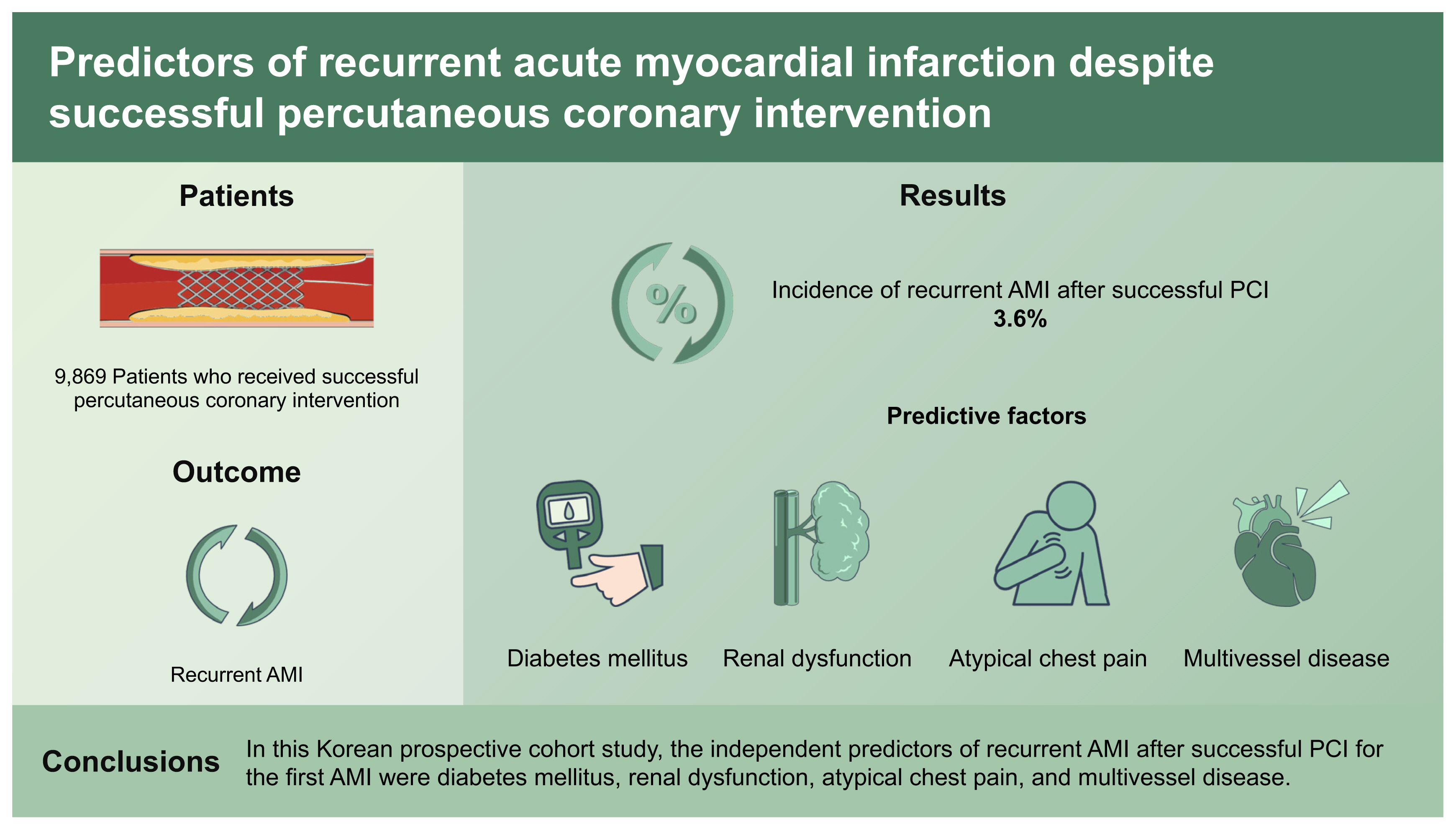
A total of 9,869 patients were included in the study (Table 1). The number of cases in the recurrent and the non-recurrent AMI groups were 359 and 9,510, respectively. The AMI recurrence rate was 3.6% (Fig. 2). Regarding the interval of recurrence, the following frequencies of reinfarction cases occurred: 25 cases within 1 day (four deaths occurred with four cardiac deaths), 46 cases within 30 days (eight deaths occurred with eight cardiac deaths), 78 cases within 6 months (nine deaths occurred with eight cardiac deaths), 56 cases within 12 months (nine deaths occurred with six cardiac deaths), 79 cases within 24 months (seven deaths occurred with five cardiac deaths), and 75 cases within 36 months (seven deaths occurred with three cardiac deaths). More detailed information about the characteristics of recurrent AMI is presented in Supplementary Table 1. Killip class III or IV was frequent in the recurrent AMI group. The presence of conditions such as hypertension, diabetes mellitus (DM), renal dysfunction, and atypical chest pain was more frequent in the recurrent AMI group than in the non-recurrent AMI group.
In both groups, the most common target lesion was the left anterior descending artery, followed by the right coronary artery, left circumflex coronary artery, and left main coronary artery (Table 2). The proportion of PCI in all locations and the frequency of multivessel disease was higher in the recurrent AMI group than in the non-recurrent AMI group. The proportion of cases in which the total ischemic time was longer than 12 hours or ejection fraction was < 50%, and lesion type B2 or C were the same in both groups. The incidence of atrial fibrillation was comparable between the groups. The ischemic burden was estimated as the maximum troponin I level, and there was no statistically significant difference between the groups. Regarding medical treatment, there was no difference between the groups in the frequency of prescribing antiplatelet agents, beta-blockers, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and statins.
The following variables with p < 0.1 in the univariable analysis were included in the multivariable logistic regression analysis after considering covariance: age ≥ 75 years, women, heart rate > 100 beats/min, Killip class III or IV, hypertension, DM, previous heart failure, smoking, renal dysfunction, atypical chest pain, PCI of the left anterior descending coronary artery, multivessel disease, and ejection fraction < 50% (Supplementary Table 2). Of these, the significant predictive factors were DM, renal dysfunction, atypical chest pain, and multivessel disease (Table 3).
To evaluate the effect of recurrent AMI, we compared MACE incidence after 1 year between patients with and without AMI recurrence using the Kaplan-Meier curve analysis. The cumulative incidence rate of MACE in the AMI recurrence group was significantly higher compared with that in the non-recurrence group (log-rank p < 0.001) (Fig. 3). Additionally, we investigated the predictors of recurrent AMI within 1 year (Supplementary Tables 3 and 4). In the multivariable analysis, the predictors of recurrent AMI within 1 year were Killip class III or IV, hypertension, DM, atypical chest pain, multivessel disease, and statin use.
This study identified several independent predictive factors for recurrent AMI after successful PCI, including DM, renal dysfunction, atypical chest pain, and multivessel disease. Given that these patients have a higher ischemic risk, clinicians should consider prescribing the dual antiplatelet therapy for a longer duration and administer more potent P2Y12 inhibitors (antiplatelet drugs) in this patient population.
DM is a well-known risk factor for the development of vascular diseases. Previous studies showed that coronary events such as restenosis and stent thrombosis were more common among patients with DM because of the modification of the response to arterial injury [13]. Therefore, adequate diabetic control is essential to prevent AMI recurrence.
According to a previous study, patients with renal dysfunction comprise a very high-risk group for coronary artery disease. In particular, the cardiovascular mortality rate of patients with end-stage renal disease treated using hemodialysis was 10 to 20 times higher than that of the general population [14]. Regarding AMI recurrence, these patients have a higher risk and need more active surveillance and cautious management.
Multivariable logistic regression analysis revealed atypical chest pain as a risk factor for AMI recurrence. Baseline characteristics of the atypical and typical chest pain groups were different. Patients presenting with atypical chest pain were older, more often women, and had longer total ischemic time; were more likely to have Killip class III or IV at admission, low systolic blood pressure, high heart rate, and renal dysfunction; were more likely to have hypertension, DM, previous cerebrovascular accident, previous heart failure, atrial fibrillation compared with those presenting with typical chest pain (Supplementary Table 5). The pathophysiology of these differences were appeared is not clear, yet. Further studies should be conducted to explore the relationship between the degree of pain and associated symptoms (e.g., dyspnea and diaphoresis), patient perception of the significance of the disease, education level, medical adherence, and to explain the pathophysiology on the risk of AMI recurrence.
Multivessel disease has been revealed a risk factor for AMI recurrence. A previous study showed that the number of stents, stenosis length, and reference diameter were associated with the risk of restenosis [15]. However, several questions need to be addressed to understand the association of multivessel disease with AMI recurrence better. First, has AMI recurred in the same vessel/vessels? Second, has AMI recurred as in-stent restenosis or in a nearby stent? Third, was there any pre-existing evidence of insignificant stenosis in the vessel at the time of the first AMI [16–18]? Finally, the length and diameter of the vessel should be considered as potential contributing factors [19,20].
A previous study evaluated the American College of Cardiology/American Heart Association lesion classification as a predictor of procedural, 30-day, and 12-month outcomes. In that study, complex lesion (type B2 and C) was associated with lower 30-day and 12-month survival rates, and the authors attributed this to the lower procedural success rate [21]. In the present study, we only enrolled patients who had successful PCI; hence, there was no association between complex lesion types and AMI recurrence.
In the multivariable analysis, the predictors of recurrent AMI within 1 and 3 years were different. The reason for the difference is not clear. Killip class III or IV, hypertension, and the use of statin were the only predictors of recurrent AMI within 1 year, and these factors can be controlled with medical treatment; however, apart from the prescription of medication, additional investigations regarding the adequacy of medical treatment and achievement of the target value should be followed-up. Furthermore, a previous study emphasized the importance of using statin to control dyslipidemia in acute coronary syndrome patients [22]. Within 1 year, which is relatively early, and after that, the stability of the plaque, medications and difference in ejection fraction between the first AMI, recurrence, and follow-up may help explain the difference of predictors. Because the high-risk patient group died within 1 year, it is thought that there might be differences in the patient characteristics starting from 1 year.
Recurrent AMI is an important risk factor for critical events such as MACE, and efforts should be taken to prevent AMI recurrence by identifying high-risk groups and their clinical characteristics; in particular, identifying modifiable risk factors is important. This will allow clinicians to implement active surveillance strategies and ultimately improve patients’ cardiac function and overall quality of life.
There are several limitations to the present study. First, because the hospitals participating in the KAMIR-NIH are the best university hospitals in Korea, patients’ clinical conditions tend to be more severe, and the quality of medical services provided is superior to those of average hospitals. Therefore, the treatment and outcomes cannot be generalized to patients treated at average hospitals. Second, multiple previous studies have demonstrated that the degree of lipid control, not only LDL-C but also non-high-density lipoprotein cholesterol, is an independent risk factor for recurrent AMI [23–25]. However, lipid profile data at the 3-year follow-up was only available for a minority of the study population (25%, 2,454 patients), which may have influenced our results. Third, we did not consider other potential factors which may influence AMI recurrence, such as the precise location of recurrent AMI compared with the first AMI location; the type of stent; number of stents; length of the stent; degree of control of other underlying diseases such as hypertension and DM; and other thrombogenic conditions such as performance status, infection, and autoimmune diseases. Finally, although we analyzed the predictive ability of several variables, we were unable to assess patient compliance accurately. This is important because another study demonstrated that good patient compliance could reduce cardiovascular event risk after AMI [26]; therefore, this aspect may have affected our results.
1. The following are independent predictors of recurrent acute myocardial infarction (AMI) after successful percutaneous coronary intervention for AMI: diabetes mellitus, renal dysfunction, atypical chest pain, and multivessel disease.
2. Patients with risk factors for AMI recurrence should undergo active surveillance and management of modifiable risk factors to prevent AMI recurrence and maintain their cardiac function and quality of life.
Acknowledgments
This study was supported by the Research of Korea Centers for Disease Control and Prevention (grant number: 2016-ER6304-02) and Chonnam National University Hospital Biomedical Research Institute (grant number: BCRI-20075).
The authors thank all the clinical investigators who contributed time and effort to this study as well as the following Korea Acute Myocardial Infarction Registry (KAMIR) investigators: Myung Ho Jeong, Youngkeun Ahn, Sung Chul Chae, Jong Hyun Kim, Seung-Ho Hur, Young Jo Kim, In Whan Seong, Donghoon Choi, Jei Keon Chae, Taek Jong Hong, Jae Young Rhew, Doo-Il Kim, In-Ho Chae, Junghan Yoon, Bon-Kwon Koo, Byung-Ok Kim, Myoung Yong Lee, Kee-Sik Kim, Jin-Yong Hwang, Myeong Chan Cho, Seok Kyu Oh, Nae-Hee Lee, Kyoung Tae Jeong, Seung-Jea Tahk, Jang-Ho Bae, Seung-Woon Rha, Keum-Soo Park, Chong Jin Kim, Kyoo-Rok Han, Tae Hoon Ahn, Moo-Hyun Kim, Ki Bae Seung, Wook Sung Chung, Ju-Young Yang, Chong Yun Rhim, Hyeon-Cheol Gwon, Seong-Wook Park, Young-Youp Koh, Seung Jae Joo, Soo-Joong Kim, Dong Kyu Jin, Jin Man Cho, Sang-Wook Kim, Jeong Kyung Kim, Tae Ik Kim, Deug Young Nah, Si Hoon Park, Sang Hyun Lee, Seung Uk Lee, Hang-Jae Chung, Jang-Hyun Cho, Seung Won Jin, Myeong-Ki Hong, Yangsoo Jang, Jeong Gwan Cho, Hyo-Soo Kim, and Seung-Jung Park.
Figure 1
Study population. The study population was derived from the nationwide prospective Korea Acute Myocardial Infarction Registry (KAMIR)-National Institutes of Health (NIH). AMI, acute myocardial infarction; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Figure 3
Cumulative rate of major adverse cardiovascular events (MACE) after 1 year from first event in acute myocardial infarction (AMI) recurred group within the 1 year vs. non-recurred group. Red line: AMI recurred group within the 1 year, Blue line: AMI non-recurred group with in the 1 year.
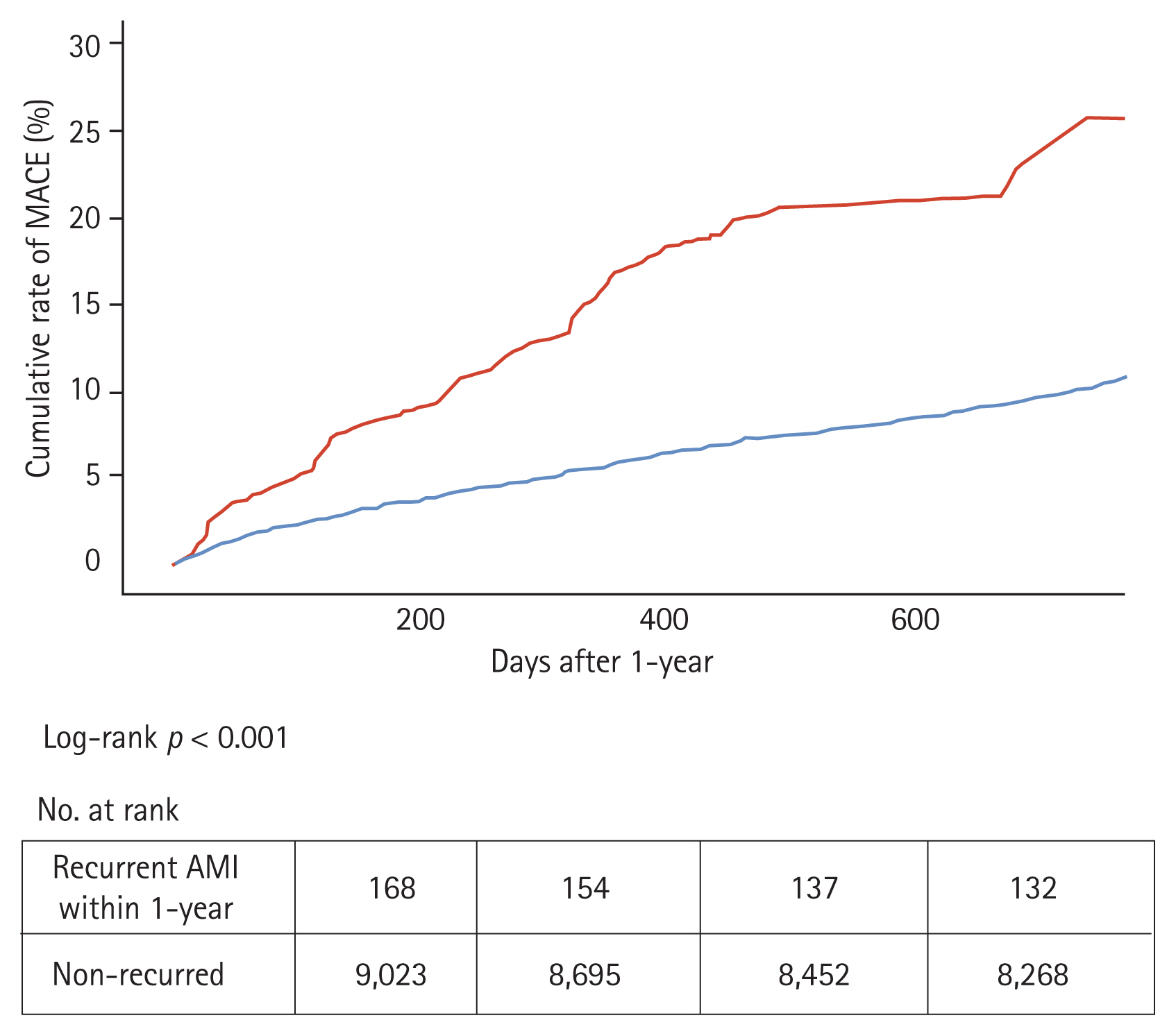
Table 1
Baseline characteristics
Table 2
Coronary angiographic, echocardiographic, laboratory findings, and medication
AMI, acute myocardial infarction; LM, left main coronary artery; LAD, left anterior descending coronary artery; LCx, left circumflex coronary artery; RCA, right coronary artery; TIMI, thrombolysis in myocardial infarction; LDL-C, low-density lipoprotein cholesterol; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Table 3
Multivariable analysis for predictors of recurrent acute myocardial infarction
REFERENCES
1. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation 2021;143:e254–e743.

2. Kim JH, Chae SC, Oh DJ, et al. Multicenter cohort study of acute myocardial infarction in Korea: interim analysis of the Korea Acute Myocardial Infarction Registry-National Institutes of Health Registry. Circ J 2016;80:1427–1436.


3. Ulvenstam G, Aberg A, Bergstrand R, et al. Recurrent myocardial infarction. 1. Natural history of fatal and non-fatal events. Eur Heart J 1985;6:294–302.


4. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012;126:2020–2035.


5. Fox KA, Dabbous OH, Goldberg RJ, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ 2006;333:1091.



6. World Health Organization. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Sydney (AU): World Health Organization, 2000;17–18.
7. Anand SS, Islam S, Rosengren A, et al. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J 2008;29:932–940.


8. Park HW, Yoon CH, Kang SH, et al. Early- and late-term clinical outcome and their predictors in patients with ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction. Int J Cardiol 2013;169:254–261.


9. Yudi MB, Clark DJ, Farouque O, et al. Trends and predictors of recurrent acute coronary syndrome hospitalizations and unplanned revascularization after index acute myocardial infarction treated with percutaneous coronary intervention. Am Heart J 2019;212:134–143.


10. Thune JJ, Signorovitch JE, Kober L, et al. Predictors and prognostic impact of recurrent myocardial infarction in patients with left ventricular dysfunction, heart failure, or both following a first myocardial infarction. Eur J Heart Fail 2011;13:148–153.


11. Song J, Murugiah K, Hu S, et al. Incidence, predictors, and prognostic impact of recurrent acute myocardial infarction in China. Heart 2020;107:313–318.



12. Nakatani D, Sakata Y, Suna S, et al. Incidence, predictors, and subsequent mortality risk of recurrent myocardial infarction in patients following discharge for acute myocardial infarction. Circ J 2013;77:439–446.


13. Aronson D, Edelman ER. Coronary artery disease and diabetes mellitus. Cardiol Clin 2014;32:439–455.



14. Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol 1998;9(12 Suppl):S16–S23.


15. Bauters C, Hubert E, Prat A, et al. Predictors of restenosis after coronary stent implantation. J Am Coll Cardiol 1998;31:1291–1298.


16. Jukema JW, Verschuren JJ, Ahmed TA, Quax PH. Restenosis after PCI. Part 1: pathophysiology and risk factors. Nat Rev Cardiol 2011;9:53–62.



17. Wihanda D, Alwi I, Yamin M, Shatri H, Mudjaddid E. Factors associated with in-stent restenosis in patients following percutaneous coronary intervention. Acta Med Indones 2015;47:209–215.

18. Sukhija R, Aronow WS, Sureddi R, et al. Predictors of in-stent restenosis and patient outcome after percutaneous coronary intervention in patients with diabetes mellitus. Am J Cardiol 2007;100:777–780.


19. Goldberg SL, Loussararian A, De Gregorio J, Di Mario C, Albiero R, Colombo A. Predictors of diffuse and aggressive intra-stent restenosis. J Am Coll Cardiol 2001;37:1019–1025.


20. Claessen BE, Smits PC, Kereiakes DJ, et al. Impact of lesion length and vessel size on clinical outcomes after percutaneous coronary intervention with everolimus-versus paclitaxel-eluting stents pooled analysis from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) and COMPARE (second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice) Randomized Trials. JACC Cardiovasc Interv 2011;4:1209–1215.

21. Theuerle J, Yudi MB, Farouque O, et al. Utility of the ACC/AHA lesion classification as a predictor of procedural, 30-day and 12-month outcomes in the contemporary percutaneous coronary intervention era. Catheter Cardiovasc Interv 2018;92:E227–E234.



22. Abtan J, Bhatt DL, Elbez Y, et al. Residual ischemic risk and its determinants in patients with previous myocardial infarction and without prior stroke or TIA: insights from the REACH Registry. Clin Cardiol 2016;39:670–677.




23. Yu X, Lu J, Li J, et al. Serum triglyceride lipase concentrations are independent risk factors for coronary artery disease and in-stent restenosis. J Atheroscler Thromb 2019;26:762–774.



24. Suzuki K, Oikawa T, Nochioka K, et al. Elevated serum non-HDL (high-density lipoprotein) cholesterol and triglyceride levels as residual risks for myocardial infarction recurrence under statin treatment. Arterioscler Thromb Vasc Biol 2019;39:934–944.


- TOOLS
-
METRICS

- Related articles
-
Current status of acute myocardial infarction in Korea2019 January;34(1)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print


