 |
 |
| Korean J Intern Med > Volume 37(6); 2022 > Article |
|
Abstract
Background/Aims
We aimed to investigate the oral beclomethasone dipropionate’s (BDP) efficacy as an add-on therapy and to clarify the predictive factor for response to oral BDP in Korean ulcerative colitis (UC) patients.
Methods
Patients with a stable concomitant drug regimen with exposure to oral BDP (5 mg/day) within 30 days before BDP initiation were included. Partial Mayo score (pMS) was used to evaluate response to oral BDP. Clinical remission (CREM) was defined as a post-treatment pMS ≤ 1 point. Clinical response (CRES) was defined as an at least 2-point decrease in post-treatment pMS and an at least 30% decrease from baseline pMS. Patients without CREM or CRES were considered nonresponders (NRs).
Results
Of all, 37 showed CREM, 19 showed CRES, and 44 were NRs. The CREM group included more patients with mild disease activity (75.7% vs. 43.2%, p = 0.011) than NRs. In contrast to NRs, CREM and CRES patients showed significant improvement of post-treatment erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) (ESR with p = 0.001, CRP with p = 0.004, respectively). Moreover, the initial rectal bleeding subscore (RBS) was significantly different between CREM and CRES, or NR (both with p < 0.001). In multivariate analyses, initial stool frequency subscore (SFS) of 0 and RBS of 0 were predictive factors for CREM (odds ratio [OR], 15.359; 95% confidence interval [CI], 1.085 to 217.499; p = 0.043 for SFS, and OR, 11.434; 95% CI, 1.682 to 77.710; p = 0.013 for RBS).
Inflammatory bowel disease (IBD) has been traditionally regarded as a disease of the Westernized countries. However, cumulative evidence of a rapid rise in the incidence and prevalence of IBD in newly industrialized countries of Asia, Eastern Europe, and South America, suggests a shift in the epidemiological pattern as a global disease [1–4]. Ulcerative colitis (UC) is a chronic immune-mediated condition of uncertain etiology afflicting the large bowel, characterized by relapsing inflammation and extension to the proximal portion in a continuous manner [5]. Diverse therapeutic options are available for UC, including 5-aminosalicylic acid (5-ASA), immunomodulators (IMMs), and biologic agents such as tumor necrosis factor (TNF) inhibitors, non-TNF inhibitors, or small molecules [6–13]. In patients with mild to moderate UC, oral systemic steroids can be considered in case of failure of therapy with an optimal dose of 5-ASA [14–17]. Corticosteroids can induce rapid remission through their well-demonstrated anti-inflammatory and immunosuppressive effects on the cellular and humoral immune systems [18,19]. However, several studies have demonstrated a wide variety of adverse events (AEs) after treatment with systemic corticosteroids, including immunosuppression, hypertension, insulin resistance, mood disorders, and suppression of the hypothalamic-pituitary-adrenal (HPA) axis [20]. Previous studies have revealed that > 90% of patients experience at least one AE [21] and that the risk of opportunistic infection synergically increases when corticosteroids are combined with other IMMs or biologic agents [22].
Owing to concerns about the adverse consequences of oral systemic corticosteroid use, topically acting oral steroids were developed. Besides the oral budesonide multimatrix system (MMX), beclomethasone dipropionate (BDP) has also been developed as a second-generation steroid with minimal systemic effects. Oral BDP is delivered in an inactive form until it reaches the distal small bowel and colon, owing to its acid-resistant film coating (Eudragit-L 100/55) that dissolves at pH < 6 [23]. After its disintegration and release in the small bowel, BDP undergoes extensive liver metabolism and fast absorption in the form of beclomethasone-17-monoproprionate, reducing the potential occurrence of AEs and thus having less effect on the systemic circulation [24,25].
In some European and non-European countries, oral BDP has been approved as an add-on therapy for patients with mild to moderate UC that is refractory to optimized 5-ASA therapy [15,26]. As oral budesonide MMX formulation is not available in Korea, BDP in the form of oral prolonged-release tablets (Clipper, Chiesi Pharmaceuticals, Parma, Italy), containing 5 mg per tablet, has been used as a topically acting oral steroid in Korea since 2010. Although previous studies including randomized controlled trials (RCTs) have demonstrated satisfactory efficacy and safety profile [27–30], real-world data about the efficacy of oral BDP as an add-on therapy to concomitant drugs in Korean patients with UC have been scarce. Therefore, in this study, we aimed to investigate the efficacy of oral BDP and to clarify the predictive factor for response to this treatment in Korean patients with UC.
This retrospective observational study enrolled patients with UC aged > 18 years who were treated with conventional drugs, such as 5-ASA, IMMs, or biologics, at Asan Medical Center, a tertiary-care hospital in South Korea, between February 2012 and October 2020. A total of 275 patients who had been exposed to oral BDP (5 mg/day) within 30 days because of acute aggravation of stool frequency or rectal bleeding subscores (RBSs) were selected. Of them, 161 patients were excluded because of modifications in the concomitant therapies, as follows: (1) oral 5-ASA within 30 days; (2) rectal therapy within 14 days; (3) IMM within 3 months; and (4) biologic therapy within 6 months. Moreover, 14 patients with exposure to systemic corticosteroids within 30 days were excluded. The remaining 100 patients with a stable regimen profile were included in this study.
The diagnosis of UC was based on conventional clinical, endoscopic, radiologic, and histopathologic criteria [5]. After 4 weeks of therapy, post-treatment disease activity was assessed using the partial Mayo score (pMS), a noninvasive 9-point scoring method consisting of the stool frequency subscore (SFS), RBS, and physician rating of disease activity (Physician’s Global Assessment [PGA]), with each category rated from 0 to 3 [31]. The definitions of clinical response (CRES) and clinical remission (CREM) for UC were adopted from the Active Ulcerative Colitis Trials [32]. CRES was defined as an at least 2-point decrease in the post-treatment pMS and an at least 30% decrease from the baseline pMS. CREM was defined as a post-treatment pMS of ≤ 1 point. Patients who did not show CRES or CREM were considered nonresponders (NRs). Change of pMS, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) were defined as post-treatment value minus pretreatment value, respectively.
All patient data were collected from the Asan IBD Registry, which has been prospectively maintained since 1997. Age, sex, date of birth, and smoking history were collected as baseline demographic data. Meanwhile, the collected clinical data were date of UC diagnosis, disease activity in pretreatment and post-treatment evaluations using the pMS, disease duration, disease distribution (E1–E3) [22], administration date of oral BDP, concomitant drug regimens, and laboratory data such as ESR, and CRP level. Ethical approval for the acquisition of data was obtained from the Institutional Review Board of Asan Medical Center (approval no. 2020–1732). Written informed consent by the patients was waived due to a retrosepctive nature of our study.
The baseline characteristics were compared between the CREM group and the NR group. Categorical variables were compared using the Fisher or chi-square test, and continuous variables were compared using the two-independent- sample t test. The Mann-Whitney U test was also used to analyze the ordinal variables. Univariate and multivariate analyses using logistic regression were performed to assess the significance of predictive factors for remission. Variables with p < 0.05 in the univariate analysis were included in the multivariate model. Regression coefficients (β), odds ratios (ORs), and the corresponding 95% confidence intervals (CIs) were calculated. The Wilcoxon signed-rank test was used to compare the pretreatment and post-treatment pMS and CRP. To compare the initial pMS subscores and change of CRP among the three groups, we used the Kruskal-Wallis test and post hoc Dunn’s multiple comparison test. Statistical significance was set at p < 0.05, except for p < 0.017 in post hoc analysis with Bonferroni Correction Method. All statistical analyses were performed using SPSS version 22.0 (IBM Corp, Armonk, NY, USA).
Table 1 shows the baseline demographic data of the patients. Of the 100 patients in total, the numbers of responders (CREM and CRES) and NRs were 56 (56%) and 44 (44%), respectively. Among the responders, 37 (66.1%) showed CREM. The median age of the total cohort at BDP initiation was 42 years (range, 32.3 to 56.0). With respect to the disease extent at BDP initiation, 14 (14%) patients had proctitis, 44 (44%) had left-sided colitis, and 42 (42%) had extensive colitis. The median disease duration at BDP initiation was 11 years (range, 6 to 17). Only one (1%) patient had severe disease activity at the commencement of oral BDP, whereas 54 (54%) had mild disease activity. In terms of the rescue therapy of each group, about half of the included patients received no further rescue therapy or retried oral BDP. Twenty-three patients needed systemic steroid treatment, 18 patients were administered systemic steroid treatment with IMM, and six patients were administered bridging systemic steroid treatment with biologic agents.
The median age at BDP initiation was significantly higher in the CREM group than in the NR group (55 years vs. 35 years, p = 0.007) (Table 2). The CREM group included more patients with mild disease activity (75.7% vs. 43.2%, p = 0.011) (Figure 1). The mean initial pMS was significantly lower in the CREM group (3.46 vs. 4.50, p = 0.014). The pretreatment and post-treatment pMS showed a significant difference in the CREM group (p < 0.001). However, in the NR group, the post-treatment pMS showed no significant change from the pretreatment pMS (p = 0.380) (Figure 2). The CREM group showed greater changes in the values of the SFS and RBS (SFS: −0.81 vs. 0.05, p < 0.001; RBS: −0.92 vs. −0.07, p < 0.001).
As shown in Supplementary Table 1, the comparison between the clinical responder and NR groups also showed similar results. The median age at BDP initiation in the responder group was 51 years, which was higher than that of the NR group with p = 0.005; moreover, larger changes in the values of the SFS and RBS were observed in the responder group (SFS: −0.86 vs. 0.05, p < 0.001; RBS: −1.0 vs. −0.07, p < 0.001). The initial pMS was higher in the NR group than the responder group, however, only numerically (p = 0.197).
In addition, 17 out of 37 patients (45.9%) with CREM had experienced clinical relapse with the median relapse of one time. The median time of relapse was 23.0 months (range, 6 to 75) and the cumulative relapse-free rates were 91.7% after 1 year and 73.0% after 2 years (Figure 3). Of the study patients, only one (1%) reported facial flushing after taking oral BDP, which was well-tolerated by most of the patients.
As shown on Table 3 and Figure 4, among responders including CREM and CRES, post-treatment ESR and CRP showed significant change from pretreatment value (ESR with p = 0.001, and CRP with p = 0.004, respectively). In contrast, there existed no significant change of post-treatment ESR and CRP in NR group (ESR with p = 0.164, and CRP with p = 0.141, respectively). The median change of ESR was 2.0 (range, −45.0 to 64.0), −2.0 (range, −35.0 to 9.0), −7.5 (range, −54.0 to 24.0) in NR, CRES, and CREM group, respectively. The median change of CRP was 0.00 (range, −3.61 to 17.4), −0.01 (range, −1.45 to 0.40), −0.01 (range, −4.9 to 0.00) in NR, CRES, and CREM group, respectively. The median change of ESR and CRP was significantly different among response groups (ESR with p = 0.001, and CRP with p = 0.008, respectively), and post hoc analysis revealed a significant difference between NR and CREM group (ESR with p = 0.004, and CRP with p = 0.016, respectively). However, both in ESR and CRP, no significant difference was demonstrated between NR and CRES group (ESR with p = 0.267, and CRP with p = 0.074), and between CRES and CREM group (ESR with p = 0.479, and CRP with p > 0.999).
As the initial pMS was significantly lower in the CREM group, the initial SFS and RBS were further investigated. In subanalyses (Figure 5), the initial SFS showed no significant difference among the three response groups (p = 0.104). In contrast, there were significant differences in the initial RBS between the CREM and CRES groups (p < 0.001) and between the CREM and NR groups (p < 0.001). However, no significant difference was observed between the CRES and NR groups (p = 0.027). In addition, all patients with CREM had an RBS of zero.
In the univariate analysis to identify the predictive factors for CREM, the significant factors included age > 40 years at BDP initiation (OR, 2.741; 95% CI, 1.102 to 6.816; p = 0.030), inital SFS of 0 (OR, 13.333; 95% CI, 1.069 to 166.374; p = 0.044), and initial RBS of 0 (OR, 7.583; 95% CI, 1.309 to 43.922; p = 0.024). Other variables such as sex, disease extent, concomitant drug regimen, and biochemical markers (e.g., elevated CRP and ESR levels at BDP initiation) were not significant factors. Consequently, initial SFS of 0 and initial RBS of 0 were significant predictive factor for CREM after 4 weeks of oral BDP in the multivariate analysis (OR, 15.359; 95% CI, 1.085 to 217.499; p = 0.043 for SFS; and OR, 11.434; 95% CI, 1.682 to 77.710; p = 0.013 for RBS) (Table 4).
Likewise, we further analyzed the predictive factors associated with response to oral BDP, considering both CREM and CRES. Although age of > 40 years at BDP initiation and an initial RBS of 0 or 1 seemed significant in univariate analyses, only initial RBS of 0 or 1 were found to be independent predictive factors of response to oral BDP (OR, 119.489; 95% CI, 12.019 to 1187.896; p < 0.001 for RBS of 0; and OR, 9.666; 95% CI, 1.078 to 86.633; p = 0.043 for RBS of 1) (Supplementary Table 2).
In the present study, approximately one-half of the patients showed CRES to oral BDP and more than one-third achieved CREM after 4 weeks of oral BDP administration. The results of the present study seem similar to those of previous studies in terms of the efficacy of oral BDP in patients with UC. According to the RCT by Rizzello et al. [28], the combination of oral BDP and 5-ASA was more effective than 5-ASA alone in patients with extensive or left-sided UC (p = 0.021 in remission). Furthermore, a multinational RCT on oral BDP administered in daily doses of 5 mg demonstrated noninferior efficacy compared with prednisolone [30]. In the meta- analysis of these RCTs, approximately 30% of patients achieved CREM after 4 weeks of treatment [33]. In addition, some studies have investigated the efficacy of oral BDP in real practice. The CREM rate in patients with mild to moderate UC was 75% in a single-center study [34]. In the RECLICU study, a retrospective, multicenter study, the CREM and CRES rates were 44.4% and 22.3%, respectively [35]. Conversely, the efficacy in our study seemed to be lower than that of a previous Korean study, which reported CRES in two-thirds and CREM in more than half of patients [36]. This discrepancy can be explained by differences in the characteristics of patients included in the two studies. The median disease duration was 5.7 years (range, 0.25 to 25) in the previous study [36] as opposed to 11 years (range, 6 to 16) in our study. Although direct comparison of past medication history among response groups is unavailable based on the presented data, 23.2% of the patients included in the previous study were concomitantly treated with azathioprine, whereas 29% of the patients included in our study had past exposure to IMMs and/or biologic agents. Given the chronic, relapsing disease course of UC, the patients in the present study may have had higher chances of exposure to other treatments, such as IMMs, corticosteroids, and biologic agents, which may have resulted in lower efficacy of oral BDP. Moreover, we included all patients with any category of a stable concomitant drug regimen. Therefore, the efficacy of oral BDP may have been underestimated compared with that in the previous study. In this regard, our study provides reasonable evidence for the choice of oral BDP as an add-on therapy in the patients who experience aggravation of symptoms despite the use of concomitant drugs.
In addition to fair clinical efficacy of oral BDP in the present study, biochemical efficacy was also investigated with change of ESR and CRP among response groups. There existed significant improvement of post-treatment ESR and CRP among responders including CREM and CRES, and the median change of both biomarkers differed significantly among response groups, especially between NR and CREM group. In the previous open-label, randomized, controlled study by Romano et al. [37], oral BDP-treated pediatric patients showed significantly decreased ESR and CRP level at 12 weeks after 8 weeks of oral BDP administration (ESR with p < 0.001, and CRP with p < 0.025). Although significant improvement of ESR and CRP was only found among responders in our study, favorable biochemical efficacy of oral BDP in adult group warrant further well-designed studies as there has been no study demonstrating the biochemical efficacy of oral BDP in adult UC patients.
Furthermore, the CREM group had a significantly higher proportion of patients with mild disease activity compared to the NR group, although there was no significant difference between the responder and NR groups. As the pMS consists of the SFS, RBS, and PGA, we performed additional analyses to identify which subscores influenced the response. Considering the subjective nature of the PGA, only the SFS and RBS were included in the subanalyses. The results revealed differences in the initial RBS among the three response groups, whereas the SFS showed no significant difference. Although statistical significance could not be assessed owing to skewness of data, all patients with CREM had an initial RBS of zero. Further, in the univariate analysis to identify the predictive factors for CREM, age > 40 years and initial SFS and RBS of 0 were significant factors; however, only initial SFS and RBS of 0 remained a significant predictive factor for CREM in the multivariate analysis. Similarly, in the RECLICU study, the CREM rate was higher in patients with mild to moderate UC than in those with severe UC [35]. Unlike the higher remission rate observed in patients with left-sided and extensive UC in the RECLI-CU study, our study found no significant difference in the response rate depending on disease extent. When deciding whether to administer oral BDP in patients with the same pMS, patients with an initial SFS or RBS of 0 are likely to have better response after 4 weeks of oral BDP. However, interim assessment of efficacy of oral BDP within week 2 or 3 after initiation was unavailable. Further studies evaluating short-term efficacy of oral BDP are warranted to help clinicians to predict clinical course during oral BDP therapy.
In terms of the safety of oral BDP, only one (1%) patient reported facial flushing after oral BDP administration and most patients showed good tolerance to the treatment. As previously mentioned, concerns about the various adverse effects of systemic corticosteroids had led to the development of second-generation corticosteroids, such as oral BDP or oral budesonide [20–22]. According to previous studies, the incidence of AEs was similar to that in the placebo and 5-ASA groups, and no serious AEs occurred [28–30]. Campieri et al. [29] reported that nine of 67 (13%) patients showed a significant reduction in mean morning plasma cortisol levels, although symptoms attributable to HPA suppression were not observed. Furthermore, a more favorable benefit-risk profile was observed in the oral BDP group, with higher response rates without steroid-related AEs, than in the prednisone group (51.2% in the oral BDP group and 37.8% in the prednisone group) [30]. The favorable safety and tolerability observed in our study can be understood in the same context as the results of previous studies.
As our study was conducted retrospectively, with post-treatment assessment after 4 weeks of therapy, the actual time required for induction of remission with oral BDP is uncertain. Among the 37 patients with CREM, only five had visited the clinic prior to the scheduled date of further assessment, and according to the records, CREM was achieved at a median of 14 days after oral BDP administration. Accordingly, although it may take a shorter time for remission, a median of 28 days of therapy was effective in all patients with CREM (data not shown). Further studies regarding the actual period of time required for induction of remission with oral BDP would be helpful for a clinical decision making in a real-world practice. In addition, 17 out of 37 patients (45.9%) experienced clinical relapse after remission. Most patients regained CREM with a few days of oral BDP, and only four (23.5%) were intractable, resulting in drug escalation to IMMs or biologics. With a median disease duration of 4.5 years, two patients had mild disease activity, whereas the other two had moderate to severe disease activity. In particular, the SFS in all four patients was over 2 points, and RBS was over 2 points in three patients. Though it was impossible to identify clinical differences among such a small number of patients, higher SFS or RBS at the time of relapse may have affected the response to oral BDP, considering the predictive factors demonstrated in this study. Collectively, considering the efficacy and safety demonstrated by oral BDP as above, we believe that it is reasonable to try oral BDP again in a mild to moderate activity of relapse. As the effect of oral BDP can be observed within a month, it can help both patients and clinicians make a careful decision before resorting to drug escalation.
The strength of our study lies in the use of real-world data on oral BDP in Korean patients with UC. Although several studies have evaluated the efficacy of oral BDP compared with conventional therapies such as 5-ASA and systemic corticosteroids, this study is the first to evaluate both clinical and biochemical efficacy of oral BDP in adult UC patients as an add-on therapy in patients who experience acute flares despite optimized therapies for maintenance. Our study found similar CRES rates to those reported in previous studies, but biochemical efficacy of oral BDP regarding ESR and CRP among adult patients was first investigated. In addition, initially mild disease activity was identified as a predictive factor for CREM in response to oral BDP. Further, the RBS, not the SFS, was revealed to be different among the CREM, CRES, and NR groups. In particular, patients with an initial RBS of zero were likely to have a favorable response to oral BDP. These results can provide guidance in the selection of appropriate candidates who are expected to show a favorable response to oral BDP as an add-on therapy among patients with UC flare-ups, thus minimizing their exposure to systemic corticosteroids.
However, besides the innate methodological limitations from the retrospective observational nature of this study, some other limitations exist. First, the small sample size precludes the generalization of our results. Given that we intended to evaluate oral BDP as an add-on therapy, patients with stable doses and regimens of all concomitant drugs were included. These inclusion criteria may have contributed to minimizing the unrecognized effects of other drugs; however, they may have also contributed to the small sample size of this study. In addition, regarding the heterogeneous profile of concomitant drug regimens among patients, certain tendencies or effects on response were difficult to identify. This, however, can be a clue for the extended role of oral BDP as an add-on therapy for patients with various concomitant drug regimens in real practice. Further studies with larger sample sizes may elucidate the effect of concomitant therapies on the response to oral BDP, enabling subgroup analysis in more homogenous settings. Second, although oral BDP is originally recommended for patients with mild to moderate disease activity according to the present guidelines for the management of UC [14,15], we also included a patient with a relatively good general condition despite having a pMS, indicating severe disease activity; however, as expected, this patient showed no response to oral BDP. Taken together, our observations indicate that oral BDP can be used for patients with mild to moderate disease activity in real practice rather than for patients with severe disease activity.
Third, we could not evaluate the effect of oral BDP on endoscopic healing and biochemical markers such as fecal calprotectin. As oral BDP was administered as a short-term rescue therapy, data on routine endoscopic examinations at the baseline and post-treatment periods were unavailable. Theoretically, a special coating polymer named Eudragit-L100/55 enables oral BDP to dissolve at pH under 5.5 to 6.0, allegedly in the distal small bowel and colon. According to a study by Nugent et al. [38], the luminal pH in the distal ileum ranges from 6.5 to 7.5, and it decreases to 5.5 to 7.5 in terminal ileum to cecum, rising to 6.1 to 7.5 in the left colon and rectum. Moreover, much lower luminal pH values were reported in patients with active disease. As we administered oral BDP in patients with active inflammation, it is conceivable that reduced intracolonic pH may help effective delivery to the affected colon, resulting in favorable efficacy in distal colon. Though it showed consistent outcomes with previous randomized controlled studies with extensive or left-sided UC patients, further studies evaluating the real efficacy of oral BDP in endoscopic healing are warranted. Likewise, although data on biochemical markers including CRP and ESR in all patients could be collected, fecal calprotectin data were seldom available in both pretreatment and post-treatment evaluations because most patients did not provide stool samples. Although the initial CRP and ESR levels showed no significant effect on response in our study, further studies investigating the relationship of fecal calprotectin and response to oral BDP are still needed.
In conclusion, oral BDP is efficacious as an add-on therapy in patients with mild to moderate UC in Korea. Regardless of any concomitant drug regimens, patients with initial SFS or RBS of 0 may be good candidates for the use of oral BDP therapy for acute UC flare-ups. Further larger, well-designed prospective studies are needed.
Figure 1
Percentage of disease activity according to clinical response. BDP, beclomethasone dipropionate.
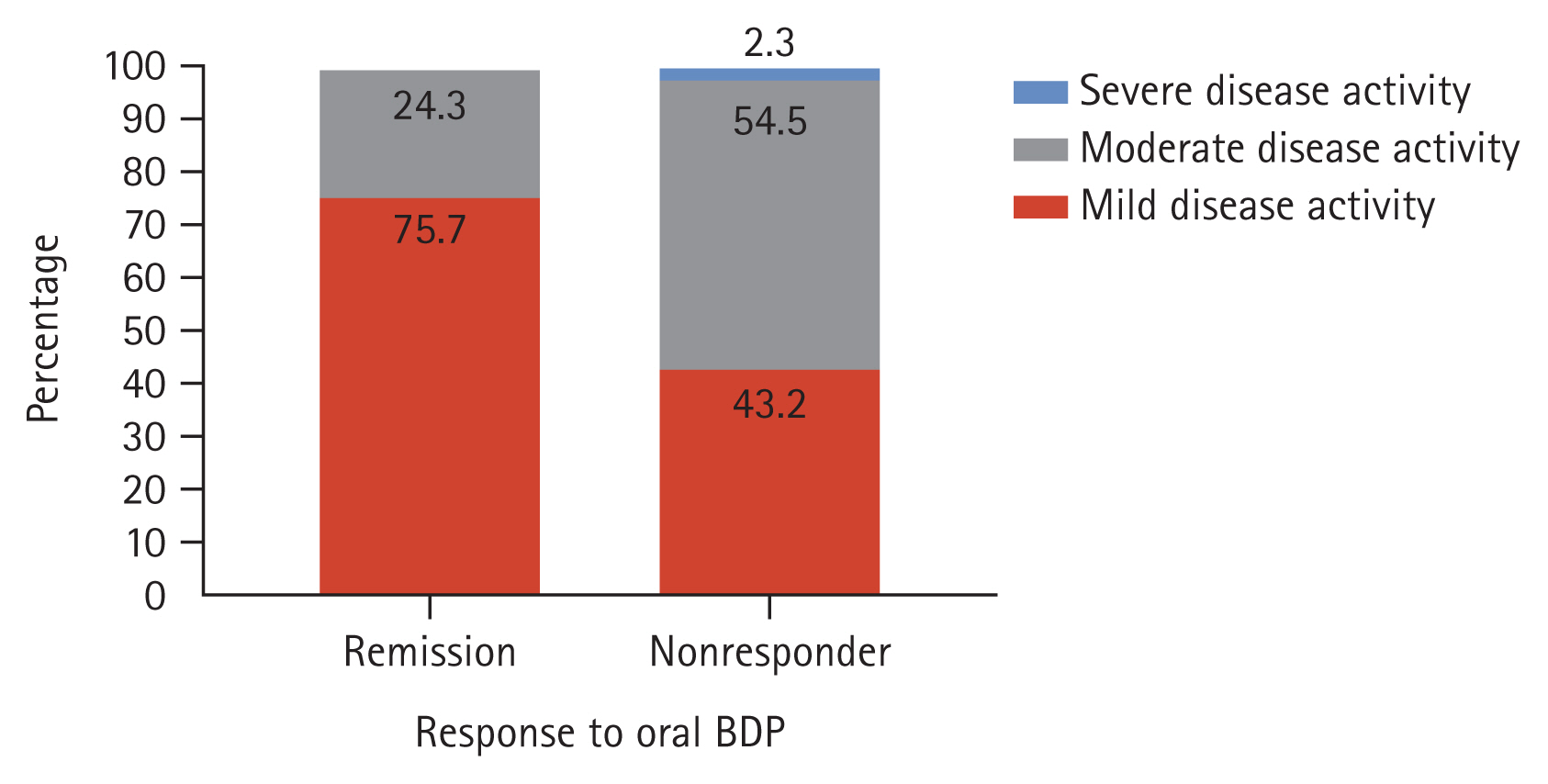
Figure 2
Pretreatment and post-treatment partial Mayo scores (pMSs) according to response to oral beclomethasone dipropionate (BDP). In the clinical remission group, the pretreatment and post-treatment pMSs showed a significant difference (p < 0.000). However, in the nonresponder group, the post-treatment pMS showed no significant change from the pretreatment pMS (p = 0.380).

Figure 3
Cumulative relapse-free rate of patients with clinical remission. In the clinical remission group, the cumulative relapse-free rate after remission were 91.7% after 1 year and 73.0% after 2 years.
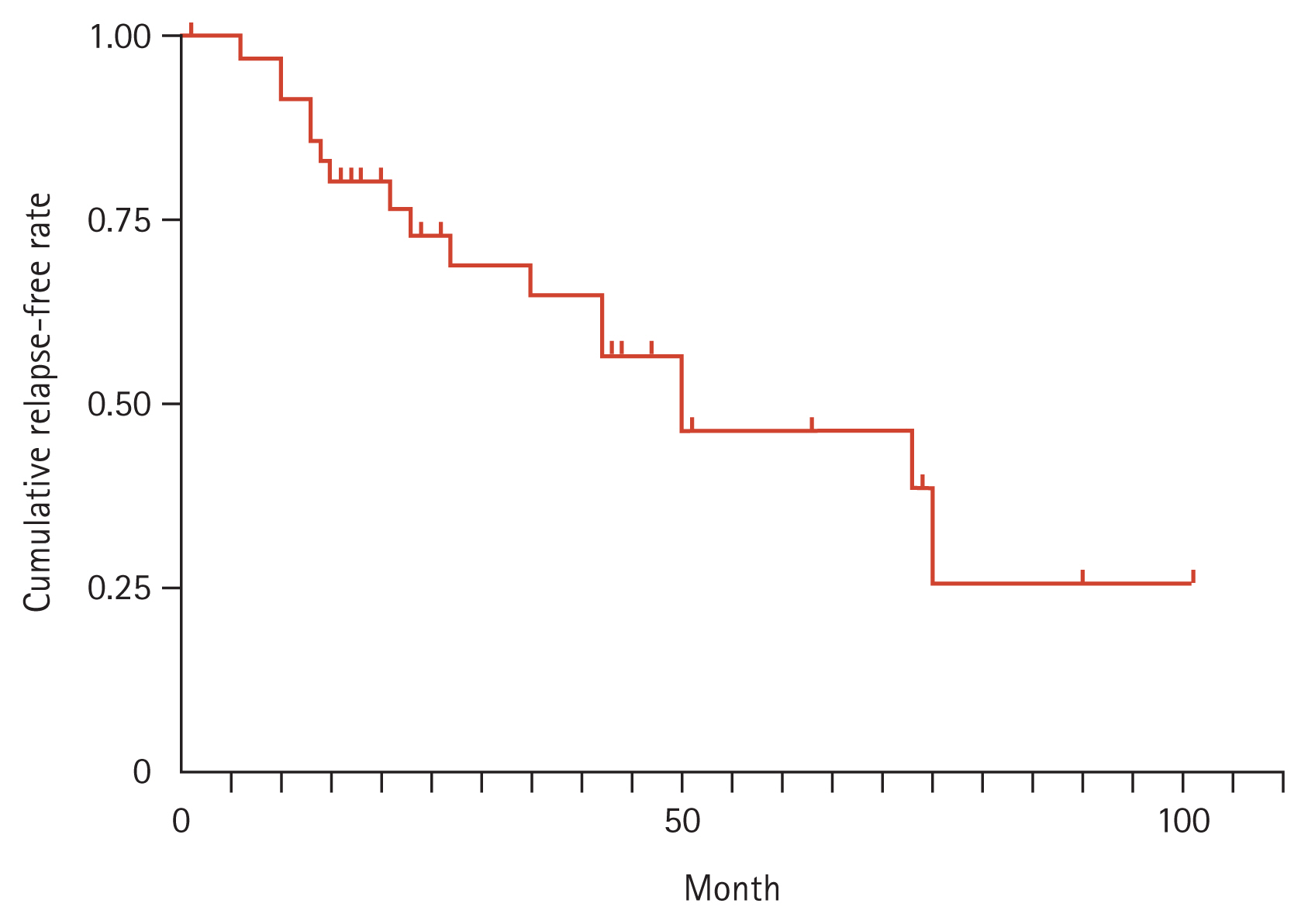
Figure 4
Pretreatment and post-treatment change of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) among response groups. In the clinical response and remission group, the pretreatment and post-treatment ESR (A) and CRP (C) showed a significant difference (ESR with p = 0.001, CRP with p = 0.004). However, in the nonresponder group, both post-treatment ESR (B) and CRP (D) had no significant change from the pretreatment value (ESR with p = 0.164, CRP with p = 0.141).

Figure 5
Kruskal-Wallis test of initial stool frequency subscore and initial rectal bleeding subscore among groups. The initial stool frequency subscore showed no significant difference among the three groups (p = 0.104). The initial rectal bleeding subscore showed significant differences between the clinical remission and clinical response groups (p < 0.001) and between the clinical remission and nonresponder groups (p < 0.001); however, no significant difference was observed between the clinical response and nonresponder groups (p = 0.027). BDP, beclomethasone dipropionate.

Table 1
Demographic and clinical characteristics of patients
Table 2
Comparison of the clinical remission and nonresponder groups
Table 3
Biochemical efficacy of oral beclomethasone dipropionate among response groups
Table 4
Univariate and multivariate logistic regression analyses for remission prediction
REFERENCES
1. Hong SW, Ye BD. The first step to unveil the epidemiology of inflammatory bowel disease in Central Asia. Intest Res 2020;18:345–346.




2. Kaibullayeva J, Ualiyeva A, Oshibayeva A, Dushpanova A, Marshall JK. Prevalence and patient awareness of inflammatory bowel disease in Kazakhstan: a cross-sectional study. Intest Res 2020;18:430–437.




3. Aniwan S, Limsrivilai J, Pongprasobchai S, et al. Temporal trend in the natural history of ulcerative colitis in a country with a low incidence of ulcerative colitis from 2000 through 2018. Intest Res 2021;19:186–193.




4. Sood A, Kaur K, Singh A, et al. Trends of inflammatory bowel disease at a tertiary care center in northern India. Intest Res 2021;19:282–290.




5. Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-based Consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017;11:649–670.


6. Bressler B, Marshall JK, Bernstein CN, et al. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology 2015;148:1035–1058.


7. Fukuda T, Naganuma M, Kanai T. Current new challenges in the management of ulcerative colitis. Intest Res 2019;17:36–44.




8. Ooi CJ, Hilmi I, Banerjee R, et al. Best practices on immunomodulators and biologic agents for ulcerative colitis and Crohn’s disease in Asia. Intest Res 2019;17:285–310.




9. Sood A, Ahuja V, Midha V, et al. Colitis and Crohn’s Foundation (India) consensus statements on use of 5-aminosalicylic acid in inflammatory bowel disease. Intest Res 2020;18:355–378.




10. Hibi T, Kamae I, Pinton P, et al. Efficacy of biologic therapies for biologic-naive Japanese patients with moderately to severely active ulcerative colitis: a network meta-analysis. Intest Res 2021;19:53–61.




11. Ooi CJ, Hilmi IN, Kim HJ, et al. Efficacy and safety of vedolizumab in ulcerative colitis in patients from Asian countries in the GEMINI 1 study. Intest Res 2021;19:71–82.




12. Shimizu H, Fujii T, Hibiya S, et al. Rapid prediction of 1-year efficacy of tofacitinib for treating refractory ulcerative colitis. Intest Res 2021;19:115–118.




13. Oh SJ, Shin GY, Soh H, et al. Long-term outcomes of infliximab in a real-world multicenter cohort of patients with acute severe ulcerative colitis. Intest Res 2021;19:323–331.




14. Harbord M, Eliakim R, Bettenworth D, et al. Third European Evidence-based Consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis 2017;11:769–784.


15. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68(Suppl 3):s1–s106.



16. Ko CW, Singh S, Feuerstein JD, et al. AGA clinical practice guidelines on the management of mild-to-moderate ulcerative colitis. Gastroenterology 2019;156:748–764.



17. Fumery M, Singh S, Dulai PS, Gower-Rousseau C, Peyrin-Biroulet L, Sandborn WJ. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol 2018;16:343–356.



18. Brattsand R, Linden M. Cytokine modulation by glucocorticoids: mechanisms and actions in cellular studies. Aliment Pharmacol Ther 1996;10:Suppl 2. 81–92.



19. Manso G, Baker AJ, Taylor IK, Fuller RW. In vivo and in vitro effects of glucocorticosteroids on arachidonic acid metabolism and monocyte function in nonasthmatic humans. Eur Respir J 1992;5:712–716.


20. Curkovic I, Egbring M, Kullak-Ublick GA. Risks of inflammatory bowel disease treatment with glucocorticosteroids and aminosalicylates. Dig Dis 2013;31:368–373.



21. Curtis JR, Westfall AO, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum 2006;55:420–426.


22. Toruner M, Loftus EV Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology 2008;134:929–936.


23. Steed KP, Hooper G, Ventura P, Musa R, Wilding IR. The in vivo behaviour of a colonic delivery system: a pilot study in man. Int J Pharm 1994;112:199–206.

24. Daley-Yates PT, Price AC, Sisson JR, Pereira A, Dallow N. Beclomethasone dipropionate: absolute bioavailability, pharmacokinetics and metabolism following intravenous, oral, intranasal and inhaled administration in man. Br J Clin Pharmacol 2001;51:400–409.




25. Roberts JK, Moore CD, Ward RM, Yost GS, Reilly CA. Metabolism of beclomethasone dipropionate by cytochrome P450 3A enzymes. J Pharmacol Exp Ther 2013;345:308–316.



26. Choi CH, Moon W, Kim YS, et al. Second Korean guidelines for the management of ulcerative colitis. Intest Res 2017;15:7–37.




27. Rizzello F, Gionchetti P, Galeazzi R, et al. Oral beclomethasone dipropionate in patients with mild to moderate ulcerative colitis: a dose-finding study. Adv Ther 2001;18:261–271.



28. Rizzello F, Gionchetti P, D’Arienzo A, et al. Oral beclometasone dipropionate in the treatment of active ulcerative colitis: a double-blind placebo-controlled study. Aliment Pharmacol Ther 2002;16:1109–1116.



29. Campieri M, Adamo S, Valpiani D, et al. Oral beclometasone dipropionate in the treatment of extensive and left-sided active ulcerative colitis: a multicentre randomised study. Aliment Pharmacol Ther 2003;17:1471–1480.



30. Van Assche G, Manguso F, Zibellini M, et al. Oral prolonged release beclomethasone dipropionate and prednisone in the treatment of active ulcerative colitis: results from a double-blind, randomized, parallel group study. Am J Gastroenterol 2015;110:708–715.



31. Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis 2008;14:1660–1666.



32. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462–2476.


33. Manguso F, Bennato R, Lombardi G, Riccio E, Costantino G, Fries W. Efficacy and safety of oral beclomethasone dipropionate in ulcerative colitis: a systematic review and meta-analysis. PLoS One 2016;11:e0166455.



34. Papi C, Aratari A, Moretti A, et al. Oral beclomethasone dipropionate as an alternative to systemic steroids in mild to moderate ulcerative colitis not responding to aminosalicylates. Dig Dis Sci 2010;55:2002–2007.



35. Nunes T, Barreiro-de Acosta M, Nos P, et al. Usefulness of oral beclometasone dipropionate in the treatment of active ulcerative colitis in clinical practice: the RECLICU Study. J Crohns Colitis 2010;4:629–636.


36. Lee YJ, Cheon JH, Kim JH, et al. Clinical efficacy of beclomethasone dipropionate in Korean patients with ulcerative colitis. Yonsei Med J 2017;58:144–149.




- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,971 View
- 197 Download
- Related articles
-
Approach to cytomegalovirus infections in patients with ulcerative colitis2021 May;36(3)
Anxiety, depression, and stress in Korean patients with chronic urticaria2020 November;35(6)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print


