 |
 |
| Korean J Intern Med > Volume 38(1); 2023 > Article |
|
Abstract
Background/Aims
Women with rheumatoid arthritis (RA) are often diagnosed with the disease during their reproductive years; however, its incidence and prevalence among women of childbearing age have not been studied. The objective of this study was to estimate the incidence and prevalence of seropositive rheumatoid arthritis (SPRA) among Korean women of childbearing age.
Methods
Women aged 20 to 44 years with SPRA were identified from National Health Insurance Service-National Health Information Database (2009 to 2016). SPRA was defined by International Classification of Diseases, 10th revision code, M05. Incidence and prevalence were calculated per 100,000 person-years and stratified by year and age.
Results
The average incidence and prevalence of SPRA from 2011 to 2016 among women of childbearing age was 24.1/100,000 person-years (95% confidence interval [CI], 23.7 to 24.5) and 105.2/100,000 person-years (95% CI, 100.9 to 109.5), respectively. The incidence increased annually from 21.0/100,000 person-years (95% CI, 20.1 to 21.9) in 2009 to 28.4 person-years (95% CI, 27.3 to 29.5) in 2016. Similarly, the prevalence increased annually from 95.7/100,000 person-years (95% CI, 93.7 to 97.6) in 2009 to 111.0 person-years (95% CI, 108.9 to 113.2) in 2015, with a slight decrease in 2016 (110.4 person-years; 95% CI, 108.2 to 112.6). The incidence and prevalence of SPRA increased with advancing age. The peak age for both incidence and prevalence of SPRA among women of childbearing age was 40 to 44 years.
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory arthritis associated with a substantial burden of functional disability. According to Global Burden of Disease Study 2017, there were almost 20 million prevalent and 1.2 million incident cases of RA globally [1]. The worldwide incidence and prevalence of RA vary greatly according to region and ethnicity; the highest prevalence and incidence is in North America and the lowest in Southeast Asia [1,2].
Despite the high regional and ethnic variation, one common feature of RA epidemiology is that the disease more frequently affects women than men. The prevalence of RA is approximately 2-fold higher in women than in men, particularly in those ≤ 70 years of age [1–4]. In recent decades, the average age of women at childbirth has significantly increased globally. It is currently ≥ 30 years [5]. Therefore, it is more likely for women to have children after a diagnosis of RA. This disease has a significant impact on childbearing and family life; impaired fertility and fecundity have been reported among women with RA [6,7]. Furthermore, many women with RA choose to limit their family size due to concerns about their children’s health and their own personal welfare [8]. Women with RA have less favorable pregnancy and offspring outcomes than unaffected women, including increased risks of intrauterine growth retardation, preeclampsia, and offspring with a smaller size for their gestational age [9,10]. High disease activity appears to be an important factor associated with impaired fertility and adverse pregnancy and offspring outcomes [11,12]. Thus, women of childbearing age with RA are a vulnerable population that require special attention.
To date, no studies have examined the incidence and prevalence of RA among women during their childbearing years. Determining theses rates nationwide may yield important insights into underlying disease biology. Additioinally, it will provide valuable information for the planning and allocation of health care resources. The presence of rheumatoid factor (RF) or anti-cyclic citrullinated peptide antibodies (ACPA) is an important predictor of disease severity [13,14]; therefore, RA incidence and prevalence determined by serological status could provide a better understanding of the risk and burden of this disease in a given population. This study aimed to estimate the incidence and prevalence of seropositive rheumatoid arthritis (SPRA), including temporal trends and age group variations, among Korean women of childbearing age using a nationwide population database.
We conducted a nationwide retrospective study using the Korean National Health Insurance Service-National Health Information Database (NHIS-NHID) from 2009 to 2016. The Korean NHIS was founded in 2000. It is a single insurer that provides coverage for 97% of the Korean population. The remaining 3% (with low incomes) are covered by the Medical Aid Program [15]. As of December 2014, the NHIS-NHID included all inpatient and outpatient claims and health care utilization data for approximately 50 million Korean people [16]. The target population in this study was women of childbearing age (defined as women between the ages of 20 to 44 years) from January 1, 2009 to December 31, 2016. We defined childbearing age as 20 to 44 years as in a study by Choi et al. [17] since half of all Korean women experience menopause between 46 and 50 years of age [18], and the Korean population census provides population statistics in age groups with 5-year-intervals. This study was approved by the Institutional Review Board of the National Health Insurance Service Ilsan Hospital (Institutional Review Board Number: NHIMC 2020-07-004) and conducted according to the principles of the Declaration of Helsinki. The database used in this study contains anonymized data for research purposes; therefore, written informed consent was not required.
In 2009, the government of the Republic of Korea subsidized medical expenses for patients with rare and intractable diseases through a copayment assistance policy called the Individual Copayment Beneficiaries Program (ICBP). SPRA was designated as a rare disease covered by this program. Under the ICBP system, the NHIS established a registration program that includes codes for the targeted disease classified by the Korean Standard Classification of Diseases (KCD)-7 (based on the International Classification of Diseases [ICD]-10), date of definitive diagnosis, and tests performed to confirm the diagnosis. In the case of RA, patients who satisfied the American College of Rheumatology (ACR) 1987 revised criteria for the classification of RA or the 2010 ACR/European League Against Rheumatism (EULAR) RA classification criteria and showed positivity for RF or ACPA were registered. To identify cases for this study, we used data from January 1, 2009 to December 31, 2016, with the assumption that all patients with SPRA have been accurately coded since 2009. We identified SPRA cases using the ICD-10 code, M05, during this period.
For incidence, the year-specific numerator was the number of patients who were incident cases in the specific calendar year. The denominator was the total mid-year population at risk. When calculating the incidence, data from January 1, 2011 to December 31, 2016, were used because data from at least 1-year prior and a washout period of at least 1 year are required to minimize the prevalent cases identified as incident cases.
For annual prevalence, the year-specific numerator was the number of prevalent cases in the specific calendar year. The denominator was the total mid-year population from the Korean National Statistical Office for that specific year. Prevalence rates were identified from 2009 to 2016. Crude rates, age-specific rates, standardized rates adjusted for age, and 95% confidence intervals (CIs) were calculated. The age-specific incidence and prevalence rate trends were similar for all years; therefore, we randomly selected the year 2013 to estimate the age specific incidence and prevalence rates because this was in the middle of the study period. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Of the 9,139,933 women aged 20 to 44 years, 13,193 were newly diagnosed with SPRA between 2011 and 2016. The average incidence was 24.1/100,000 person-years (95% CI, 23.7 to 24.5).
Table 1 shows the trend in incidence per year. The incidence of SPRA increased annually from 21.0/100,000 person-years (95% CI, 20.1 to 21.9) in 2011 to 28.4 person-years (95% CI, 27.3 to 29.5) in 2016 among women of childbearing age.
To analyze the trends in incidence according to age, we calculated the age-specific incidence rate for 2013 in women of childbearing age. The incidence of SPRA increased with advancing age; it was lowest in the 20 to 24 years age group (1.3/100,000 person-years; 95% CI, 0.8 to 1.9) and highest in the 40 to 44 years age group (28.3/100,000 person-years; 95% CI, 26.0 to 30.5). The steepest increase in incidence was observed between the 30 and 34 years (13.9/100,000 person-years; 95% CI, 12.2 to 15.5) and 35–39 years (22.9/100,000 person-years; 95% CI, 20.7 to 25.0) age groups (Fig. 1A).
From 2009 to 2016, 24,590 women between 20 and 44 years of age had a diagnosis of SPRA. The average prevalence of SPRA in women in their childbearing years was 105.2/100,000 person-years (95% CI, 100.9 to 109.5).
Table 2 shows the trend in prevalence by year. In 2009, the prevalence of SPRA in women of childbearing age was 95.7/100,000 person-years (95% CI, 93.7 to 97.6). A yearly increase in prevalence was observed thereafter, with a peak prevalence of 111.0/100,000 person-years (95% CI, 108.9 to 113.2) in 2015. In 2016, the prevalence slightly decreased to 110.4/100,000 person-years (95% CI, 108.2 to 112.6), compared with the peak prevalence in 2015.
The age-specific prevalence rate in 2013 was calculated as the prevalence in different age groups of women in their childbearing years (Fig. 1B). An increase in the prevalence of SPRA with increasing age with the lowest prevalence in the 20 to 24 years age group (5.3/100,000 person-years; 95% CI 4.1 to 6.4). The highest prevalence was in the 40 to 44 years age group (230.0/100,000 person-years; 95% CI, 223.6 to 236.4). The steepest increase in prevalence was observed between the 35 and 39 years age group (143.6/100,000 person-years; 95% CI, 138.2 to 148.9) and 40 and 44 years age group (230.0/100,000 person-years; 95% CI, 223.6 to 236.4).
This is the first nationwide population-based, epidemiologic study of SPRA among women of childbearing age in South Korea using the NHIS-NHID, which includes health information from nearly the entire Korean population. Our study found a yearly increase in the incidence and prevalence rates of SPRA in women of childbearing age. This reflects the significant risk and burden of disease in this particular population group.
The average of incidence and prevalence rates of SPRA among Korean women of childbearing age were 24.1/ 100,000 person-years and 266.8/100,000 person-years, respectively. The incidence and prevalence of RA according to serological status have not been previously studied in Korea. One nationwide study in Korea including cases of SPRA and seronegative RA has shown an incidence of 28.5/100,000 person-years in the total population, 44.3/100,000 person-years in women of all ages, and 12.8/100,000 person-years in men of all ages [19]. The prevalence was 0.29% in the total population, 0.46% in women of all ages, and 0.12% in men of all ages in 2010 [19]. Approximately 85% of patients with RA have RF or ACPA in Korea [20,21]; therefore, the average incidence and prevalence of SPRA in women of childbearing age found in our study is comparable to those in the total Korean population. Furthermore, this is only slightly lower than the incidence in Korean women of all ages. This suggests that women of childbearing age are a high-risk population for SPRA and that a high disease burden is imposed on this particular population. The incidence and prevalence rates of SPRA in women of childbearing age were not analyzed in previous studies in different geographical regions. However, we can infer from the Global Burden of Disease study (2017) that women of childbearing age have comparable incidence and prevalence rates with those of the total population [1]. More studies are required to accurately evaluate the risk and burden of SPRA in women of childbearing age.
The incidence and prevalence of RA is dynamic; it varies over time and among different geographical regions [22–27]. Despite variation in incidence and prevalence estimates, the overall annual rates of RA have increased globally from 1990 to 2017 by 8.2% and 7.4%, respectively [1]. The prevalence of RA in Korea has increased from 0.28% in 2009 to 0.32% in 2012 [19]. However, the incidence of RF-positive RA has declined in European populations between 1980 and 2000 [28]. Furthermore, it has declined in Olmsted County, Minnesota, USA from 2005 to 2014 compared with the previous decade [29]. Additionally, a decline in the prevalence of RF-positive RA has been reported in Pima Indians from 1986 to 1995 when compared with previous decades. Potential reasons for this decreased incidence are unclear; however, changes in environmental factors, such as smoking and obesity, may have contributed to this decline.
Similarly, a decline in SPRA in European populations has been reported in conjunction with a decline in cigarette smoking [30,31]. In a population-based study, obesity was associated with anti-CCP negative RA, especially in women [32]. In contrast to global trends of decreased incidence and prevalence of RF-positive RA, we found that the incidence and prevalence of SPRA increased annually from 2011 to 2015 among women of childbearing age. This increased incidence and prevalence appears to coincide with a significant increase in the prevalence of smoking in women aged 19 to 34 years [33]. This suggests that environmental factors, such as cigarette smoking, increase the risk and burden of SPRA among women of childbearing age as reported in the total population. Seropositivity in RA is predictive of a more aggressive disease and poor long-term prognosis [34]; therefore, more efforts should be made to correct controllable environmental factors, especially in Korean women of childbearing age, to decrease the risk and burden of the disease in this population group.
We found an increased incidence and prevalence of SPRA with advancing age in women of childbearing age. This finding is expected as it has been shown globally in both male and female populations, with a peak incidence and prevalence in the age groups of sixth and seventh decades of life and decreasing thereafter [1,9,28]. However, when we analyzed age-specific incidence and prevalence in detail by estimating these measures in 5-year intervals, we found that the steepest increase in incidence was observed between 30–34 and 35–39 years age groups. Interestingly, the steepest increase in prevalence was observed between the 35–39 and 40–44 years age groups. The average age at which Korean women give birth was 32.2 years for their first child, 33.8 years for their second child, and 35.2 years for their third child. This is the highest among Organization for Economic Co-operation and Development (OECD) countries according to statistics from 2019 [35]. Taken together, these data indicate that it is highly likely for women with SPRA to give birth after a diagnosis of RA. Many studies have shown that women with RA are an at-risk population for adverse pregnancy and offspring outcomes [9,10,36]; lower birth rates are demonstrated in women with RA [8]. Interestingly, we found decreased delivery rates in Korea women of childbearing age with SPRA (data not shown). Therefore, the additional burden of adverse pregnancy, offspring outcomes, and smaller family size is expected in Korean women of childbearing age.
We have recently reported the incidence and prevalence of systemic lupus erythematosus (SLE) among Korean women of childbearing age using the same NHIS-NHID database [37]. Although we found that risks of SLE and SPRA are high in women during their childbearing years and this population bears a significant burden of both diseases, a noteworthy difference was observed between the epidemiology of SPRA and SLE. The incidence of SLE from 2011 to 2016 showed minimal year on year variation. Moreover, the peak age of incidence coincided with the period of time when they are most likely to have children [37]. This finding suggested that the risk of developing SLE is mainly affected by intrinsic factors such as age, gender, and female sex hormones. However, we found increased incidence of SPRA during 2011 to 2016, which coincided with an increase in the prevalence of smoking in women aged 19 to 34 years in Korea. This suggests that environmental factors, such as cigarette smoking, increase the risk of SPRA among women of childbearing age. The recognition of epidemiologic differences in SLE and SPRA among women of childbearing age is important for the planning and allocating health resources to decrease the risks of these diseases in this particular population. Health care spending in Korea is concentrated on children and elderly; these populations receive more copayment benefits when compared with out-of-pocket expenditures. Additionally, medical costs are high in the elderly due to multiple associated comorbidities [38]. The ratio of medical expenses to insurance premium was 14.82 for children aged 0 to 6 years and 7.04 for seniors aged > 65 years. By contrast, the ratio was 0.46 for adults aged 19 to 39 years [38]. RA is a chronic disease requiring lifelong treatment and many comorbidities develop over its course; therefore, allocating heath expenses in women of childbearing age would decrease the risk of RA development and may contribute to a reduction in the long-term total health care costs in Korea.
Despite these important findings, this study has some limitations. First, we defined SPRA cases based on ICD-10 codes; therefore, the diagnosis may have been inaccurate. To minimize this problem, we only included NHIS-NHID data starting from 2009 since all patients with SPRA were registered based on the 1987 ACR or 2010 ACR/EULAR RA classification criteria with an accurate diagnostic code in order to receive benefits from the government through a newly launched individual co-payment program for rare and intractable diseases that was started in the second half of the year of 2008. Second, as NHIS-NHID data are limited to information on patients who visited health institutions, the prevalence and incidence rates might have been underestimated. However, this may have only slightly impacted our results because the NHIS is a single insurer that covers the entire population of Korea. Nevertheless, this is the first study to define the incidence and prevalence rates of SPRA among women of childbearing age, a unique population group with a high disease burden.
In conclusion, the risk of SPRA is high in women during their childbearing years. Therefore, this population bears a significant disease burden. The findings of this study may serve as benchmark data for the planning and allocation of health care resources to provide tailored maternal and child health care and education services for this particular population group. Moreover, it may contribute to reducing the burden and risk of RA on women of childbearing age. Further research assessing the functional burden, such as disability-adjusted life years, and economic burden of SPRA are required to further elucidate the overall burden of disease in this age group.
1. The incidence and prevalence rates of seropositive rheumatoid arthritis in women of childbearing age is increasing yearly, which reflects a significant risk and burden of disease in this particular population.
2. The findings of this study may serve as benchmark data for planning and allocation of health care resources to provide tailored maternal and child health care and education services for this particular population group and may contribute to reducing the burden and risk of rheumatoid arthritis on women of childbearing age.
Acknowledgments
This work was supported by the National Health Insurance Service Ilsan Hospital grant (2018-20-002). This study used the NHIS-NHID (NHIS 2021-1-667), which was created by the NHIS. The authors alone are responsible for the content and writing of the paper.
Figure 1
(A) Age-specific incidence and (B) age-specific prevalence in 2013 for rheumatoid arthritis in women of childbearing age (20 to 44 years).
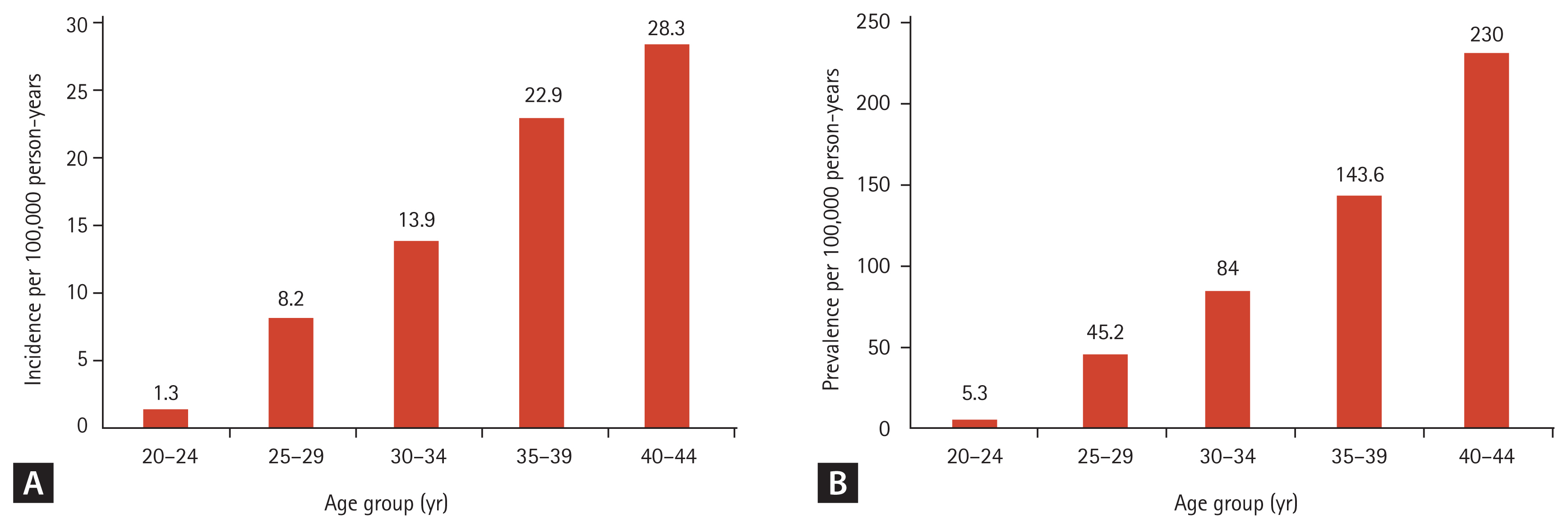
Table 1
Incidence of rheumatoid arthritis in women of childbearing age (20 to 44 years) by year from 2011 to 2016
Table 2
Prevalence of rheumatoid arthritis in women of childbearing age (20 to 44 years) by year from 2009 to 2016
REFERENCES
1. Safiri S, Kolahi AA, Hoy D, et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis 2019;78:1463–1471.


2. Rudan I, Sidhu S, Papana A, et al. Prevalence of rheumatoid arthritis in low- and middle-income countries: a systematic review and analysis. J Glob Health 2015;5:010409.


3. Cross M, Smith E, Hoy D, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1316–1322.


4. Moradi-Lakeh M, Forouzanfar MH, Vollset SE, et al. Burden of musculoskeletal disorders in the Eastern Mediterranean Region, 1990–2013: findings from the Global Burden of Disease Study 2013. Ann Rheum Dis 2017;76:1365–1373.

5. Organization for Economic Cooperation and Development. OECD Family Database [Internet] Paris (FR): OECD, Social Policy Division, 2019. [cited 2022 Mar 31]. Available from: http://www.oecd.org/els/family/database.htm
.
6. Nelson JL, Koepsell TD, Dugowson CE, Voigt LF, Daling JR, Hansen JA. Fecundity before disease onset in women with rheumatoid arthritis. Arthritis Rheum 1993;36:7–14.


7. Wallenius M, Skomsvoll JF, Irgens LM, et al. Fertility in women with chronic inflammatory arthritides. Rheumatology (Oxford) 2011;50:1162–1167.


8. Katz PP. Childbearing decisions and family size among women with rheumatoid arthritis. Arthritis Rheum 2006;55:217–223.


9. Smeele H, Dolhain R. Current perspectives on fertility, pregnancy and childbirth in patients with Rheumatoid Arthritis. Semin Arthritis Rheum 2019;49:S32–S35.


10. Norgard BM, Larsen MD, Friedman S, Knudsen T, Fedder J. Decreased chance of a live born child in women with rheumatoid arthritis after assisted reproduction treatment: a nationwide cohort study. Ann Rheum Dis 2019;78:328–334.


11. de Man YA, Hazes JM, van de Geijn FE, Krommenhoek C, Dolhain RJ. Measuring disease activity and functionality during pregnancy in patients with rheumatoid arthritis. Arthritis Rheum 2007;57:716–722.


12. Brouwer J, Hazes JM, Laven JS, Dolhain RJ. Fertility in women with rheumatoid arthritis: influence of disease activity and medication. Ann Rheum Dis 2015;74:1836–1841.


13. van der Heijde DM, van Leeuwen MA, van Riel PL, van de Putte LB. Radiographic progression on radiographs of hands and feet during the first 3 years of rheumatoid arthritis measured according to Sharp’s method (van der Heijde modification). J Rheumatol 1995;22:1792–1796.

14. Ziegelasch M, Boman A, Martinsson K, et al. Anti-cyclic citrullinated peptide antibodies are associated with radiographic damage but not disease activity in early rheumatoid arthritis diagnosed in 2006–2011. Scand J Rheumatol 2020;49:434–442.


15. Song SO, Jung CH, Song YD, et al. Background and data configuration process of a nationwide population-based study using the Korean national health insurance system. Diabetes Metab J 2014;38:395–403.



16. Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci 2017;32:718–728.




17. Choi WI, Jeong J, Lee DY, Shim HY, Lee CW. Cesarean delivery may be protective against neoplasms of the uterine cervix in women of childbearing age. Eur J Cancer Prev 2020;29:501–503.


18. Chae HD, Choi SY, Cho EJ, et al. Awareness and experience of menopausal symptom and hormone therapy in Korean postmenopausal women. J Menopausal Med 2014;20:7–13.



19. Won S, Cho SK, Kim D, et al. Update on the prevalence and incidence of rheumatoid arthritis in Korea and an analysis of medical care and drug utilization. Rheumatol Int 2018;38:649–656.



20. Sung YK, Cho SK, Choi CB, et al. Korean Observational Study Network for Arthritis (KORONA): establishment of a prospective multicenter cohort for rheumatoid arthritis in South Korea. Semin Arthritis Rheum 2012;41:745–751.


21. Min HK, Kim HR, Lee SH, et al. Four-year follow-up of atherogenicity in rheumatoid arthritis patients: from the nationwide Korean College of Rheumatology Biologics Registry. Clin Rheumatol 2021;40:3105–3113.



22. Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum 2010;62:1576–1582.



23. Jacobsson LT, Hanson RL, Knowler WC, et al. Decreasing incidence and prevalence of rheumatoid arthritis in Pima Indians over a twenty-five-year period. Arthritis Rheum 1994;37:1158–1165.


24. Gabriel SE, Crowson CS, O’Fallon WM. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955–1985. Arthritis Rheum 1999;42:415–420.


25. Shichikawa K, Inoue K, Hirota S, et al. Changes in the incidence and prevalence of rheumatoid arthritis in Kamitonda, Wakayama, Japan, 1965–1996. Ann Rheum Dis 1999;58:751–756.



26. Hochberg MC. Changes in the incidence and prevalence of rheumatoid arthritis in England and Wales, 1970–1982. Semin Arthritis Rheum 1990;19:294–302.


27. Pedersen JK, Svendsen AJ, Horslev-Petersen K. Incidence of rheumatoid arthritis in the southern part of Denmark from 1995 to 2001. Open Rheumatol J 2007;1:18–23.



28. Kaipiainen-Seppanen O, Kautiainen H. Declining trend in the incidence of rheumatoid factor-positive rheumatoid arthritis in Finland 1980–2000. J Rheumatol 2006;33:2132–2138.

29. Myasoedova E, Davis J, Matteson EL, Crowson CS. Is the epidemiology of rheumatoid arthritis changing? Results from a population-based incidence study, 1985–2014. Ann Rheum Dis 2020;79:440–444.



30. Abhishek A, Doherty M, Kuo CF, Mallen CD, Zhang W, Grainge MJ. Rheumatoid arthritis is getting less frequent-results of a nationwide population-based cohort study. Rheumatology (Oxford) 2017;56:736–744.



31. Pedersen M, Jacobsen S, Klarlund M, et al. Environmental risk factors differ between rheumatoid arthritis with and without auto-antibodies against cyclic citrullinated peptides. Arthritis Res Ther 2006;8:R133.



32. Wesley A, Bengtsson C, Elkan AC, et al. Association between body mass index and anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis: results from a population-based case-control study. Arthritis Care Res (Hoboken) 2013;65:107–112.


33. Chang Y, Kang HY, Lim D, Cho HJ, Khang YH. Long-term trends in smoking prevalence and its socioeconomic inequalities in Korea, 1992–2016. Int J Equity Health 2019;18:148.




34. Berglin E, Johansson T, Sundin U, et al. Radiological outcome in rheumatoid arthritis is predicted by presence of antibodies against cyclic citrullinated peptide before and at disease onset, and by IgA-RF at disease onset. Ann Rheum Dis 2006;65:453–458.



35. Statistics Korea. Birth statistics in 2019 [Internet] Daejeon (KR): Statistics Korea Press, 2020. [cited 2022 Mar 31]. Available from: http://kostat.go.kr/portal/eng/pressReleases/8/10/index.board?bmode=read&bSeq=&aSeq=385158&pageNo=1&rowNum=10&navCount=10&currPg=&searchInfo=&sTarget=title&sTxt=
.
36. Kishore S, Mittal V, Majithia V. Obstetric outcomes in women with rheumatoid arthritis: results from Nationwide Inpatient Sample Database 2003–2011. Semin Arthritis Rheum 2019;49:236–240.


37. Chung MK, Park JS, Lim H, Lee CH, Lee J. Incidence and prevalence of systemic lupus erythematosus among Korean women in childbearing years: a nationwide population-based study. Lupus 2021;30:674–679.



38. National Health Insurance Service. National Health Insurance Service Statistics [Internet] Wonju (KR): National Health Insurance Service, 2019. [cited 2022 Mar 31]. Available from: https://www.nhis.or.kr/nhis/together/wbhaec06700m01.do?mode=view&articleNo=138284
.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



