 |
 |
| Korean J Intern Med > Volume 37(3); 2022 > Article |
|
This article has been corrected. See Korean J Intern Med. 2022 Nov 01; 37(6): 1269.
Abstract
Background/Aims
The study investigated the incidence of thromboembolic events (TEE) in head and neck (H&N) cancer patients who received concurrent chemoradiotherapy (CCRT) with cisplatin, and analyzed the factors affecting TEE occurrence
Methods
Two hundred and fifty-seven patients who started CCRT with cisplatin for H&N cancer from January 2005 to December 2019 were analyzed.
While malignant tumor cells induce hypercoagulation through several mechanisms, the administration of chemotherapeutic agents may be associated with thrombotic events [1,2]. As a result, the risk of thromboembolic events (TEE) in cancer patients is about four times higher than in the general population, and that risk increases up to 6.5 times for patients receiving chemotherapy [3,4]. In particular, TEE incidence in various cancer types of patients treated with chemotherapy including cisplatin, one of the most frequently used agents, was reported to be higher compared to those with other regimens [5ŌĆō9]. Moreover, TEE occurrence may affect cancer patients outcomes [10,11].
Head and neck (H&N) cancer refers to malignancy, usually squamous cell carcinoma, in areas such as the nasopharynx, nasal cavity, oropharynx, oral cavity, hypopharynx, and larynx, and incidence is relatively high in many countries [12,13]. In locally advanced H&N cancer, concurrent chemoradiotherapy (CCRT) with cisplatin is the established standard therapy for definitive treatment and adjuvant therapy [14ŌĆō17]. Nonetheless, there are small number of reports regarding the incidence of TEE in H&N cancer with conflicting results, without any real-world study which investigated TEE in patients receiving CCRT with cisplatin [18ŌĆō20].
For that reason, we investigated the incidence of TEE in H&N cancer patients who received CCRT with cisplatin, and analyzed the factors affecting TEE occurrence.
To begin, all patients who started CCRT with cisplatin for H&N cancer between January 2005 and December 2019 were retrospectively identified. The eligibility criterion was diagnosis with H&N squamous cell carcinoma, including other types of carcinoma in cases of nasopharyngeal cancer. Patients who underwent CCRT with cisplatin as definitive therapy following diagnosis or at the time of local recurrence as well as adjuvant therapy after surgical resection, were included in the analysis.
Exclusion criteria included patients with non-squamous histology except for nasopharyngeal cancer; those with distant metastases other than cervical lymph nodes; those who experienced TEE within 3 months prior to initiation of CCRT; those who underwent chemotherapy such as induction therapy before CCRT; those who received other anticancer drugs in addition to cisplatin during CCRT; and those who had started CCRT at other hospitals before continuing of treatment at our institution. Patients with history of chemotherapy for H&N cancer or other types of cancer were included, with the exception of those who had previously received platinum agents (cisplatin, carboplatin, oxaliplatin).
The study protocol was approved by the Ajou University Hospital Institutional Review Board (IRB No. AJIRB-MED-MDB-20-456). The informed consent was waived by the IRB.
Patient information was collected retrospectively from medical records and radiological reports, including age, gender, smoking history, previous TEE history, cancer site, cancer stage, Eastern Cooperative Oncology Group performance status at the start of CCRT, purpose of CCRT, interval of cisplatin administration, radiation dose, Khorana score, and occurrence of TEE [21,22]. Tumor stage classification was based on the American Joint Committee on Cancer 8th edition [23].
For the purpose of this study, TEE was defined as deep vein thrombosis (DVT), pulmonary embolism (PE), other types of venous thrombosis, myocardial infarction, cerebral artery thrombosis, and other types of arterial thrombosis, that occurred during or within 6 months of completion of CCRT. Confirmation of TEE occurrence was based on patient radiological reports, including computed tomography, magnetic resonance imaging, Doppler ultrasonography, and coronary angiography.
During CCRT, cisplatin was usually administered, with a dosage of 100 mg/m2 every 3 weeks for 3 cycles or 30 mg/m2 weekly for 7 weeks. The radiation treatment dose was 200 cGy per day, with a target of 7,000 cGy at 35 fractions. Treatment dose and schedule were modified at the discretion of the treating physicians.
The Khorana score is a model that predicts the risk of venous thromboembolism (VTE) associated with chemotherapy [22]. The score is composed of five clinical and laboratory variables: site of cancer (very high-risk site, 2 points; high-risk site, 1 point); pretreatment leukocyte count (more than 11 ├Ś 109/L); pretreatment hemoglobin (less than 10 g/dL) and/or use of erythropoiesis stimulating agents; pretreatment platelet count (more than 350 ├Ś 109/L); and body mass index (BMI) of 35 kg/m2 or more (all 1 point each). H&N cancer is assigned a score of 0 at the cancer site.
Continuous variables are presented as mean ┬▒ standard deviation and categorical variables as frequencies and percentages. Comparisons of continuous and categorical variables were performed by Mann-Whitney test and FisherŌĆÖs exact test, respectively. The cumulative incidences of TEE were calculated using the Nelson-Aalen cumulative hazard estimator in R software version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). All statistical analyses were two-sided and performed using SPSS version 23.0 for Windows (IBM Co., Armonk, NY, USA) except for the cumulative incidences of TEE.
Of the 265 H&N cancer patients who underwent CCRT with cisplatin during the study period, 257 patients were included in the analysis while eight patients were excluded for the following reasons: previous history of chemotherapy including platinum (four patients); history of TEE within 3 months before the start of treatment (two patients); salivary gland adenocarcinoma (one patient); and induction chemotherapy before CCRT (one patient).
Table 1 summarizes the clinical characteristics of all patients and those with occurrence of TEE. Of the 257 eligible patients, 224 (87.2%) were under the age of 70 years, with a median age of 59 (range, 18 to 78), and 219 (85.2%) were male. The most prevalent cancer site was the nasopharynx (72 patients/28.6%), and the most common stage was IV (124 patients/48.2%). Forty-one patients were followed for less than 6 months after completion of CCRT for the following reasons: death (19 patients); transfer to other hospitals (13 patients); and lost to follow-up (nine patients).
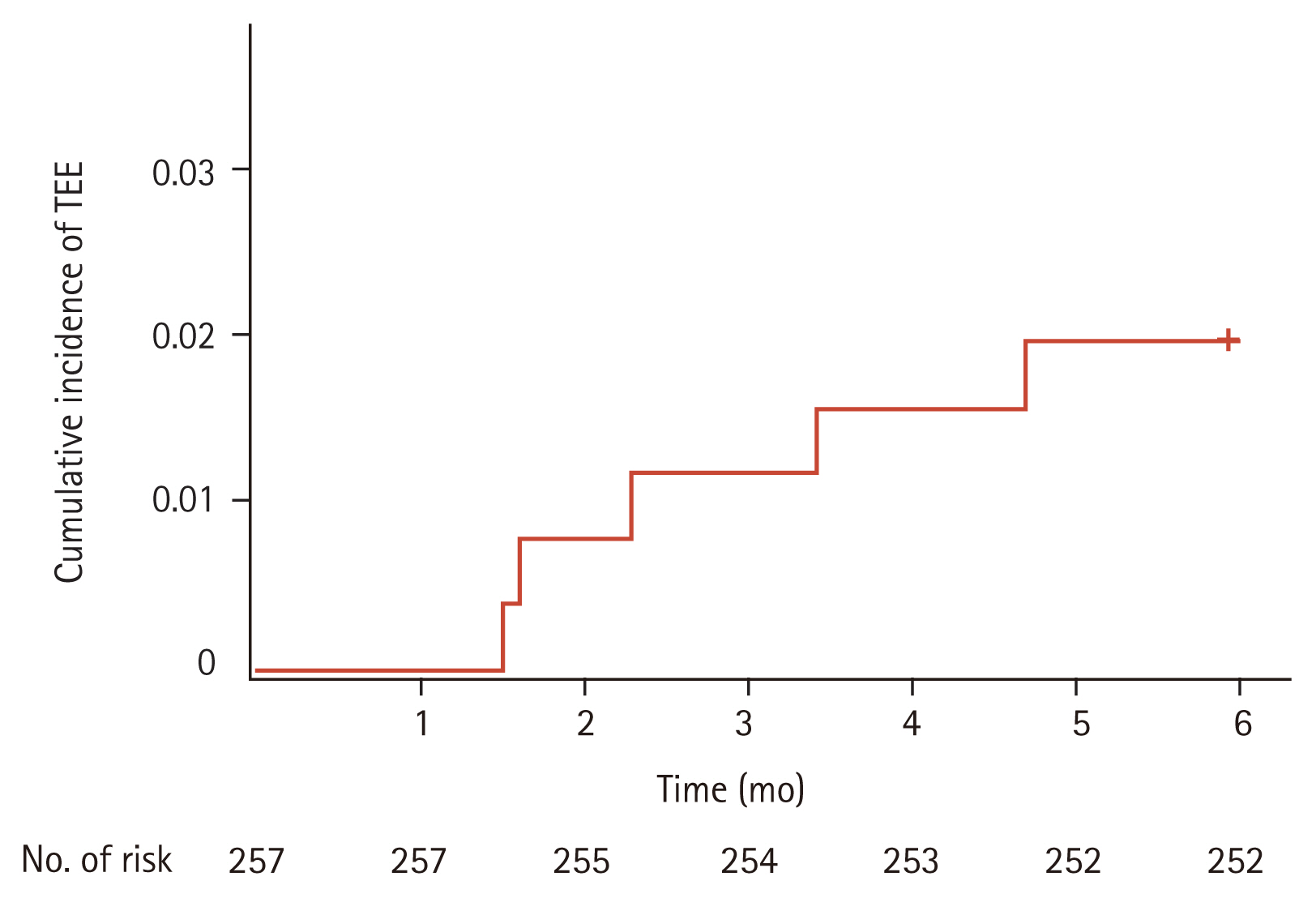
TEE occurred in five patients (1.9%), with no significant association between the incidence of TEE and clinical characteristics. Among patients with TEE occurrence, Khorana score were 0 (two patients), 1 (one patient), and 2 (two patients), with a significant correlation between TEE and Khorana score (p = 0.010) (Table 1). Cumulative incidence rates of TEE were 0.8% at the 2nd month, 1.6% at the 4th month, and 1.9% at the 6th month, respectively (Fig. 1). Types of TEE included DVT alone (three patients/60%), simultaneous occurrence of DVT and PE (one patient/20%), and arterial thrombosis (common carotid artery) (one patient/20%). No TEE event was diagnosed after progression or recurrence of disease.
To our knowledge, the present study is the first to investigate the incidence of TEE in H&N cancer patients who underwent CCRT with cisplatin in real-world practice. The observed incidence of TEE was 1.9%, and Khorana score was the only factor associated with the risk of TEE.
In cancer patients, various risk factors increase the incidence of TEE. Among patient-related factors, TEE is known to be associated with age, obesity, thrombocytosis, leukocytosis, hemoglobin < 10 g/dL before chemotherapy, and a history of previous TEE [22,24]. Treatment-related factors such as chemotherapy are also associated with risk of TEE [2].
Conflicting results have been reported regarding the incidence of TEE in H&N cancer patients. According to one meta-analysis, the incidence of VTE in H&N cancer patients was 0.16% to 3.13% [18]. Incidence of VTE in H&N cancer patients after surgery is generally low (0% to 8%), although one study reported higher rate of 26.3% [18,20]. While one study reported that H&N cancer is the least common type of cancer diagnosed following VTE, another large study identified H&N cancer as the second highest risk for VTE among 18 types of carcinoma [18,19]. In a large population-based study, the incidence of TEE in non-cancer population was 1.4%, which is similar to the rate of occurrence among H&N cancer patients reported in several studies [18,25].
In the present study, the observed incidence rate of TEE was 1.9%, which is not that dissimilar to the rate in the general population and aligns with previous studies regarding the incidence of TEE in H&N cancer [18]. However, most previous studies of TEE in H&N cancer analyzed heterogenous populations consisting mainly of a surgical treatment alone group, with small number of reports about patients who received chemotherapy including various regimens [18,20]. The present study investigated patients who underwent CCRT with cisplatin, which is the essential chemotherapeutic agent for management of H&N cancer. Although the mechanism of cisplatin-induced hypercoagulability is not entirely clear, several studies have proposed possible pathophysiologic processes; these include impaired autoregulation of the vascular system, an altered balance between thrombosis and fibrinolysis, direct endovascular damage, increased procoagulant activity of red blood cells, and modulation of tissue factor on human monocytes [26ŌĆō28].
There have been several reports of increased TEE incidence in patients treated with cisplatin-based regimens. Among these, Moore et al. [8] reported an 18.1% incidence of TEE related to cisplatin-based chemotherapy in 932 patients with various types of malignancy. The reported incidence of TEE in non-small cell lung cancer patients who underwent a cisplatin-based regimen was 8.0% to 17.6%, with 10.2% of the 1-year incidence rate of TEE in small cell lung cancer patients treated with platinum-based chemotherapy [5ŌĆō7,29]. In the present study, the incidence of TEE in H&N cancer patients during or after CCRT with cisplatin seems to be low when compared to patients with other types of cancer who received cisplatin-based regimens. A larger-scale study is required to determine the possible explanation for these findings.
In the present study, one interesting result is the significant association between the TEE occurrence and Khorana score despite of the low incidence of TEE itself. As a means of predicting the risk of chemotherapy-associated thrombosis in cancer patients, the Khorana scoring system has some limitations. For example, since BMI > 35 kg/m2 is rare among Asian patients, accurate evaluation is difficult [5,30]. In the present study cohort, only one patient (0.4%) had a BMI of > 35 kg/m2, which figure was not significantly different from that of the previous Asian study [5,30]. Nonetheless, Khorana score was the only factor associated with the TEE incidence in present study. Therefore, in cases of H&N cancer patients with high Khorana score receiving cisplatin-CCRT, treating physician should be aware of possible TEE occurrence during or for a period of time after CCRT. In addition, these patients should be informed about the somewhat higher risk of TEE before starting treatment.
The present study has several limitations. First, as a retrospective study based on a review of medical records, selection bias may occur, resulting in possible underestimation of TEE incidence. Second, the small number of patients with TEE means that statistically significant results are less reliable, especially in terms of the relationship between TEE incidence and clinical characteristics. Third, we did not compare TEE incidence in patients who underwent CCRT as adjuvant therapy, (i.e., almost 50% of the total patients,) with incidence in those who received adjuvant radiotherapy alone flowing surgical resection. Finally, the present study cohort comprised patients from a single institution over a fairly long period. Nevertheless, this study seems to be clinically meaningful because it investigated all patients during the defined period who underwent CCRT with cisplatin, which is the mainstay of treatment for locally advanced H&N cancer, reflecting real-world clinical practice in Korea.
1. The incidence of thromboembolic event (TEE) in head and neck cancer patients who underwent concurrent chemoradiotherapy with cisplatin was relatively low (1.9%) when compared to other types of cancer.
2. Patients with a high Khorana score require more careful surveillance for possible occurrence of TEE.
Table┬Ā1
Patients characteristics and their relationship with thromboembolic events
| Characteristic | Total | TEE incidence | p value | |
|---|---|---|---|---|
| Non-TEE cases | TEE cases | |||
| Total patients | 257 (100) | 252 | 5 | |
| Age, yr | 0.125 | |||
| ŌĆā< 70 | 224 (87.2) | 221 (87.7) | 3 (60.0) | |
| ŌĆāŌēź 70 | 33 (12.8) | 31 (12.3) | 2 (40.0) | |
| Gender | 0.554 | |||
| ŌĆāMale | 219 (85.2) | 215 (85.3) | 4 (80.0) | |
| ŌĆāFemale | 38 (14.8) | 37 (14.7) | 1 (20.0) | |
| Cancer sites | 0.169 | |||
| ŌĆāNasopharynx | 72 (28.0) | 72 (28.6) | 0 | |
| ŌĆāNasal cavity | 10 (3.9) | 9 (3.6) | 1 (20.0) | |
| ŌĆāOropharynx | 72 (28.0) | 69 (27.4) | 3 (60.0) | |
| ŌĆāOral cavity | 35 (13.6) | 34 (13.5) | 1 (20.0) | |
| ŌĆāHypopharynx | 35 (13.6) | 35 (13.9) | 0 | |
| ŌĆāLarynx | 30 (11.7) | 30 (11.9) | 0 | |
| ŌĆāSalivary gland | 1 (0.4) | 1 (0.4) | 0 | |
| ŌĆāUnknown primary site | 2 (0.8) | 2 (0.8) | 0 | |
| Cancer stagea | 0.626 | |||
| ŌĆāI | 21 (8.2) | 21 (8.3) | 0 | |
| ŌĆāII | 36 (14.0) | 36 (14.3) | 0 | |
| ŌĆāIII | 47 (18.3) | 47 (18.7) | 0 | |
| ŌĆāIV | 124 (48.2) | 120 (47.6) | 4 (80.0) | |
| ŌĆāLocal recurrence | 29 (11.3) | 28 (11.1) | 1 (20.0) | |
| ECOG performance state | 1.000 | |||
| ŌĆā0,1 | 235 (91.4) | 230 (91.3) | 5 (100.0) | |
| ŌĆā2,3 | 22 (8.6) | 22 (8.7) | 0 | |
| Smoking history | 0.176 | |||
| ŌĆāNo | 115 (44.7) | 111 (44.0) | 4 (80.0) | |
| ŌĆāYes | 142 (55.3)b | 141 (56.0) | 1 (20.0) | |
| TEE history | 1.000 | |||
| ŌĆāNo | 240 (93.4) | 235 (93.3) | 5 (100.0) | |
| ŌĆāYes | 17 (6.6) | 17 (6.7) | 0 | |
| Purpose of CCRT | 0.677 | |||
| ŌĆāDefinitive treatment | 132 (51.4) | 130 (51.6) | 2 (40.0) | |
| ŌĆāAdjuvant CCRT | 125 (48.6)c | 122 (48.4) | 3 (60.0) | |
| Cisplatin interval | 0.283 | |||
| ŌĆā3 weeks interval | 203 (79.0) | 200 (79.4) | 3 (60.0) | |
| ŌĆāWeekly | 54 (21.0) | 52 (20.6) | 2 (40.0) | |
| Cumulative radiation dose, cGy | 6,458.0 ┬▒ 843.1 | 6,455.6 ┬▒ 850.8 | 6,580.0 ┬▒ 238.7 | 0.677 |
| Khorana score | 0.010 | |||
| ŌĆā0 | 198 (77.0) | 196 (77.8) | 2 (40.0) | |
| ŌĆā1 | 50 (19.5) | 49 (19.4) | 1 (20.0) | |
| ŌĆā2 | 8 (3.1) | 6 (2.4) | 2 (40.0) | |
| ŌĆā3 | 1 (0.4) | 1 (0.4) | 0 | |
REFERENCES
1. Falanga A, Russo L, Milesi V, Vignoli A. Mechanisms and risk factors of thrombosis in cancer. Crit Rev Oncol Hematol 2017;118:79ŌĆō83.


3. Heit JA, Silverstein MD, Mohr DN, Petterson TM, OŌĆÖFallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000;160:809ŌĆō815.


4. Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005;293:715ŌĆō722.


5. Lee YG, Lee E, Kim I, et al. Cisplatin-based chemotherapy is a strong risk factor for thromboembolic events in small-cell lung cancer. Cancer Res Treat 2015;47:670ŌĆō675.



6. Mellema WW, van der Hoek D, Postmus PE, Smit EF. Retrospective evaluation of thromboembolic events in patients with non-small cell lung cancer treated with platinum-based chemotherapy. Lung Cancer 2014;86:73ŌĆō77.


7. Numico G, Garrone O, Dongiovanni V, et al. Prospective evaluation of major vascular events in patients with nonsmall cell lung carcinoma treated with cisplatin and gemcitabine. Cancer 2005;103:994ŌĆō999.


8. Moore RA, Adel N, Riedel E, et al. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol 2011;29:3466ŌĆō3473.



9. Seng S, Liu Z, Chiu SK, et al. Risk of venous thromboembolism in patients with cancer treated with cisplatin: a systematic review and meta-analysis. J Clin Oncol 2012;30:4416ŌĆō4426.


10. Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med 2006;166:458ŌĆō464.


11. Kuderer NM, Francis CW, Culakova E, et al. Venous thromboembolism and all-cause mortality in cancer patients receiving chemotherapy. J Clin Oncol 2008;26(15_suppl):9521.

12. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359ŌĆōE386.


14. Porceddu SV, Campbell B, Rischin D, et al. Postoperative chemoradiotherapy for high-risk head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2004;60:365ŌĆō373.


15. Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004;350:1945ŌĆō1952.


16. Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003;21:92ŌĆō98.


17. Forastiere AA, Zhang Q, Weber RS, et al. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol 2013;31:845ŌĆō852.


18. Haen P, Mege D, Crescence L, Dignat-George F, Dubois C, Panicot-Dubois L. Thrombosis risk associated with head and neck cancer: a review. Int J Mol Sci 2019;20:2838.


19. Paneesha S, McManus A, Arya R, et al. Frequency, demographics and risk (according to tumour type or site) of cancer-associated thrombosis among patients seen at outpatient DVT clinics. Thromb Haemost 2010;103:338ŌĆō343.


20. Kakei Y, Akashi M, Hasegawa T, Minamikawa T, Usami S, Komori T. Incidence of venous thromboembolism after oral oncologic surgery with simultaneous reconstruction. J Oral Maxillofac Surg 2016;74:212ŌĆō7.


21. Sorensen JB, Klee M, Palshof T, Hansen HH. Performance status assessment in cancer patients: an inter-observer variability study. Br J Cancer 1993;67:773ŌĆō775.



22. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008;111:4902ŌĆō4907.



23. Lydiatt WM, Patel SG, OŌĆÖSullivan B, et al. Head and neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:122ŌĆō137.


24. Kroger K, Weiland D, Ose C, et al. Risk factors for venous thromboembolic events in cancer patients. Ann Oncol 2006;17:297ŌĆō303.


25. Khorana AA, Dalal M, Lin J, Connolly GC. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 2013;119:648ŌĆō655.


26. Lu CF, Yu HJ, Hou JX, Zhou J. Increased procoagulant activity of red blood cells in the presence of cisplatin. Chin Med J (Engl) 2008;121:1775ŌĆō1780.


28. Walsh J, Wheeler HR, Geczy CL. Modulation of tissue factor on human monocytes by cisplatin and adriamycin. Br J Haematol 1992;81:480ŌĆō488.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



