 |
 |
|
|
|
Abstract
Background/Aims
We compared the efficacy of the granisetron transdermal system (GTS) with that of ondansetron for controlling chemotherapy-induced nausea and vomiting (CINV) in patients treated with highly emetogenic chemotherapy (HEC).
Methods
We randomized a total of 389 patients to groups treated by GTS and ondansetron before HEC. The primary endpoint was the percentage of patients achieving complete response (CR; no retching/vomiting/rescue medication) of CINV from the time of chemotherapy initiation to 24 hours after the last administration of chemotherapy (prespecified non-inferiority margin of 15%). Quality of life (QoL) was also assessed using the Functional Living Index-Emesis (FLIE).
Results
The per protocol analysis included 152 (47.80%) and 166 patients (52.20%) in the GTS and ondansetron groups, respectively. In the full analysis set, the most common diagnosis, regimen, and period of chemotherapy were lung cancer (149 patients, 40.27%), cisplatin-based regimen (297 patients, 80.27%), and 1 day chemotherapy (221 patients, 59.73%). The CR rates were 86.84% and 90.36% in the GTS and ondansetron groups, respectively; the treatment difference was ŌłÆ3.52% (95% confidence interval, ŌłÆ10.52 to 3.48) and met the primary endpoint, indicating that GTS was not inferior to ondansetron. Patient satisfaction, assessed on the FLIE, showed significantly higher scores in the GTS group compared to the ondansetron group (mean ┬▒ standard deviation, 1,547.38 ┬▒ 306.00 and 1,494.07 ┬▒ 312.05 mm, respectively; p = 0.0449).
Chemotherapy-induced nausea and vomiting (CINV) constitutes one of the most unpleasant complications in cancer treatments, and can lead to electrolyte imbalances, dehydration, decreased appetite, and impaired performance status. Thus, CINV can delay scheduled treatments. Of the neurotransmitters responsible for CINV, 5-hydroxytryptophan receptor type 3 (5-HT3) is particularly important in the pathophysiology of acute nausea and vomiting [1]. Conventional 5-HT3 receptor antagonists (RAs), including ondansetron, dolasetron, palonosetron, and granisetron are recommended for treating CINV in patients receiving chemotherapy with moderate to high emetogenic potential. The triple combination of aprepitant, a neurokinin-1 receptor antagonist (NK-1 RA), with one of the 5-HT3 RAs and dexamethasone achieved a better protective effect in both acute and delayed CINV [2].
Granisetron transdermal system (GTS) is a well-known formulation of 5-HT3 RAs, containing 34.3 mg granisetron in the adhesive layer, and developed to slowly release 3.1 mg of granisetron, mean plasma concentration of 2.2 ng/mL per 24 hours for up to 5 days [3]. The efficacy of GTS was verified in the management of CINV and compared with classical treatment routes such as oral or intravenous (IV) administration of granisetron in patients receiving multiday, moderately emetogenic chemotherapy (MEC) or highly emetogenic chemotherapy (HEC) [4,5]. Moreover, a comparative clinical study determined that the efficacy of GTS was non-inferior to that of second-generation 5-HT3 RAs, such as palonosetron, in MEC [6]. However the appropriate use of 5-HT3 RA in a triple drug combination for HEC has not yet been determined through randomized trials.
Therefore, we compared the efficacy and tolerability of multiday GTS for the prevention of CINV in patients receiving HEC regimens with ondansetron, one of the most commonly used 5-HT3 RAs in Korea.
This study was a multicenter, randomized, open-label, parallel-group, active-controlled, phase IV trial conducted at seven centers in the Republic of Korea. The primary objective was to determine whether the performance of GTS was non-inferior to that of ondansetron in patients receiving multiday HEC. Secondary objectives were to evaluate safety, tolerability, the adhesive properties of the GTS, and patient-reported satisfaction with efficacy in controlling CINV. Written informed consent was acquired from all participants before study enrollment. The present study was approved by the Institutional Research Ethics Board of Seoul St. MaryŌĆÖs Hospital and Uijeongbu St. MaryŌĆÖs Hospital of the Catholic University of Korea (No. XCMIMV0041S and XCMIMV0041U), and registered on the ClinicalTrials.gov registry (identifier: NCT01659775).
Criteria for inclusion included age Ōēź 20 years, life expectancy of Ōēź 3 months, and an Eastern Cooperative Oncology Group status of Ōēż 2. Patients scheduled to receive one cycle of chemotherapy (for Ōēż 5 days with HEC) were eligible for study enrollment. Both chemotherapy-na├»ve and chemotherapy-treated patients were eligible for study inclusion. In patients receiving multiday chemotherapy, the HEC had to be administered on day 1. Exclusion criteria were hypersensitivity of skin to patches; contraindications to 5-HT3 RA; any other cause that can induce nausea and vomiting except CINV; radiation therapy to the brain, abdomen, or whole body within 7 days of study entry; any nausea, retching, or uncontrolled vomiting within 72 hours before the beginning of chemotherapy; and clinically significant abnormalities on electrocardiogram and/or baseline corrected QTc prolongation. The concomitant medications were permitted, except for drugs that control symptoms of brain metastasis, brain tumor, or seizure disorders; selective serotonin-reuptake inhibitor (SSRI) antidepressants (unless a stable dose was used during the study period); drugs that can prolong the QTc interval, any dopamine RA, NK-1 RA, or 5-HT3 RA; and any other investigational drug.
After screening for enrollment, patients were randomized 1:1 to receive either the GTS or ondansetron by using a scratch card-based randomization system. The random allocation code was generated by an independent statistician. Sex, chemotherapy regimen, and being chemotherapy naïve were included as stratification factors. Chemotherapy was classified as cisplatin or non-cisplatin containing regimens, with each regimen having achieved at least level 5 of emetogenicity per Hesketh classification [7].
In the GTS group, because of the slow transdermal delivery of 3.1 mg of granisetron per 24 hours, it needs minimal 24 hours to reach mean plasma concentration of 2.2 ng/mL for proper efficacy of antiemesis, the patient or the investigator applied a single GTS to dry, intact skin on the lateral aspect of the upper arm at least 24 to 48 hours before the administration of the first chemotherapeutic agent, and kept it in place for 120 hours following commencement of the first chemotherapy. In the ondansetron group, patients received 24 or 32 mg IV ondansetron for 15 minutes before the first chemotherapeutic agent was administered on day 1, and, thereafter, took 8 mg ondansetron orally twice a day on days 2 to 5. All patients received aprepitant 125 mg orally once on day 1, 1 hour prior to chemotherapy, and then 80 mg once a day on days 2 and 3; and dexamethasone 12 mg IV once on day 1, 30 minutes prior to chemotherapy, and then 8 mg orally once a day on days 2 through 4 in both groups. Rescue drugs including metoclopramide, lorazepam, dexamethasone chosen by investigators were permitted for any grade of breakthrough CINV after infusion of the first chemotherapeutic agent in each group.
The primary efficacy endpoint was the percentage of patients achieving complete remission of CINV (complete response [CR], no retching/vomiting, and no use of rescue medication) during the primary endpoint evaluation period (PEEP; from chemotherapy initiation until 24 hours after the last chemotherapy administration). Secondary endpoints of efficacy included the percentage of patients achieving CR of CINV per study day (days 1, 2, 3, 4, and 5) and the overall study period (days 1 to 5); percentage of patients achieving complete control (CC; no more than mild nausea, no retching/vomiting, and no use of rescue medication) during the PEEP; and CC of CINV per study day and the overall study period. Severity was assessed based on patient-reported scores using a 4-point scale (0 for none, 1 for mild, 2 for moderate, and 3 for severe) for nausea and 5-point scale (0 for none, 1 for mild, 2 for moderate, 3 for severe, and 4 for life-threatening) for vomiting per study day and during the overall study period, respectively.
The adhesiveness percentage of the GTS (only for patients in the GTS group; 0, Ōēź 90% adhesion area; 1, 75% to 90% adhesion area; 2, 50% to 75% adhesion area; 3, < 50% adhesion area, but not detached; and 4, patch detached) and the frequencies and grades of breakthrough CINV (from the Common Terminology Criteria for Adverse Events [CTCAE] version 4.0) were recorded by patients in a diary together with the use of rescue antiemetics at the end of each day during the 5-day study period. The GTS was not applied again even though the adhesiveness percentage below 100% during study period. In addition, 120 hours after chemotherapy initiation, patients were instructed to rate their satisfaction according to the grade of control of CINV and affected quality of life (QoL) during the study period by using the Functional Living Index-Emesis (FLIE) scale [8]. Questions in the index specifically addressed the impact of CINV on social and emotional function, physical activities, and ability to enjoy meals. The severity of CINV was recorded on the visual analog scale (VAS), which ranged from ŌĆ£not at allŌĆØ (0 mm) to ŌĆ£a great dealŌĆØ (100 mm) in response to each statement; the 18-item instrument contained nine items each for nausea and vomiting, and the possible total score ranged from 0 to 1,800 mm. Higher scores indicated a more positive impact on the patientŌĆÖs functional living due to CINV.
The non-inferiority margin (╬ö) was 15%, as defined in previous non-inferiority studies of 5-HT3 RA in CINV [3,8]. Assuming a weighted CR of 68.8% with ondansetron [9ŌĆō13] and a difference between treatment groups of Ōēż 15% (╬ö), 300 patients were required to complete the study to ensure 80% statistical power. We planned random allocation of 177 patients to each group to account for a predicted dropout rate of 15%. If the lower limit of the two-sided 95% confidence interval (CI) of this point estimate was greater than ŌłÆ╬ö, the hypothesis of non-inferiority was accepted. The statistical significance of differences in secondary endpoints was calculated using descriptive statistics evaluating the frequency of nausea and retching/vomiting and VAS in FLIE. A two-sample t test or the Wilcoxon rank-sum test was performed to evaluate continuous data from both groups. Categorical data were assessed by the chi-square test or FisherŌĆÖs exact test. We performed statistical analyses using the safety set (SS), the full analysis set (FAS), and the per protocol set (PPS). The definitions of each set are as follows: SS, all patients who had Ōēź 1 dose of treatment regimen of study; FAS, all SS patients who received Ōēź 1 efficacy assessment; PPS, all FAS patients who did not have any protocol violations that directly affected or impinged upon the primary endpoint. Because this was a non-inferiority study, we performed the primary efficacy analysis in the PPS. All analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
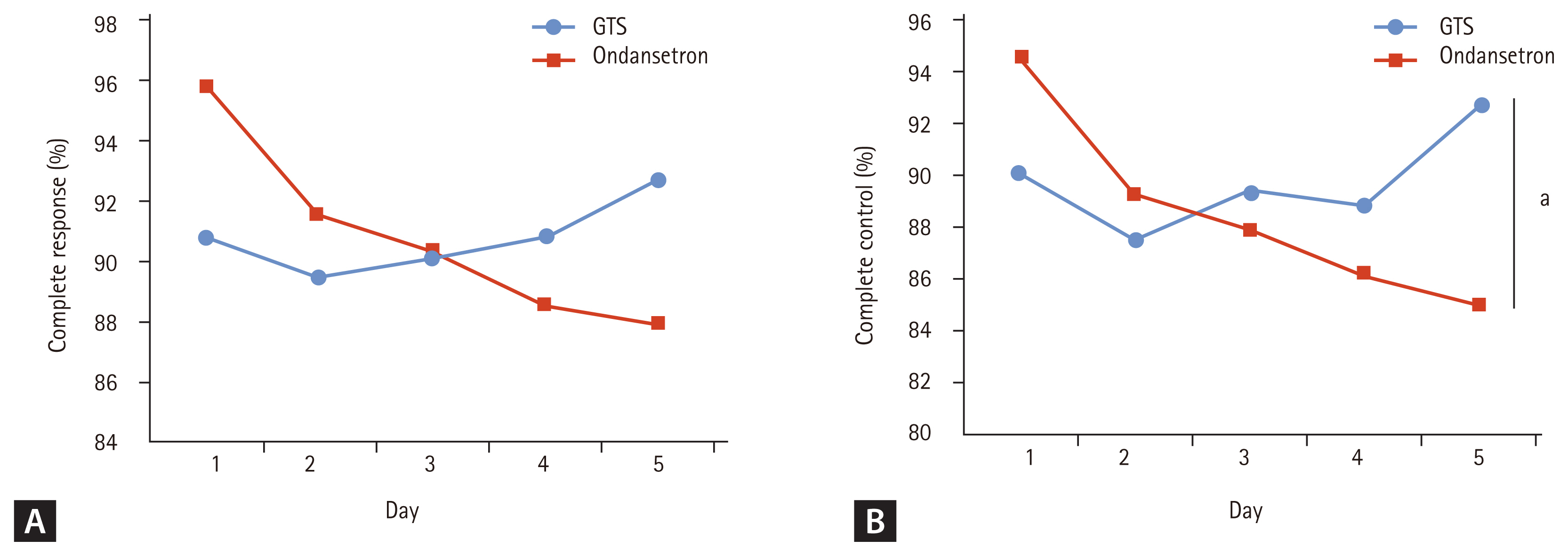
From August 2011 to November 2012, 392 patients were assessed for eligibility, and 389 patients were randomized to the GTS (n = 199) and ondansetron (n = 190) groups. Of these, 381 were included in the SS, 370 in the FAS, and 318 in the PPS. Fig. 1 shows the reasons for study withdrawal. Baseline demographic characteristics were similar between both groups, including stratification variables, as shown in Table 1.
In the PPS, 132 patients (86.84%) of the GTS group and 150 patients (90.36%) of the ondansetron group achieved CR of CINV during the PEEP showed in Table 2; the estimated between-group difference was ŌłÆ3.52% (95% CI, ŌłÆ10.52 to 3.48). The lower limit of the 95% CI of the difference between the GTS and ondansetron was greater than ŌłÆ15%, indicating that the GTS was non-inferior to ondansetron, and met the predefined primary endpoint. In the subgroup analysis by stratification factors including sex, chemotherapy regimen, and being chemotherapy na├»ve, the CR rate of the PPS was comparable and was not significantly different between the GTS and ondansetron treatment in any subgroup.
The CR and CC rates during the overall study period and the CC rate during the PEEP were not significantly different between the GTS and ondansetron groups (p > 0.05). Assessments of the CR and CC per day are presented in Fig. 2. The CR and CC rates on the first day were higher in the ondansetron group (90.79% [n = 138] vs. 95.78% [n = 159] for CR, p = 0.0733; and 90.13% [n = 137] vs. 94.58% [n = 157] for CC, p = 0.1337). However, the CR rates became higher from the fourth and CC from the third day in the GTS group, respectively. On the fifth day, the differences of the CR and CC rates between the GTS and ondansetron group became greater and the difference of the CC rate was statistically significant (p = 0.0278).
Overall severity of nausea and retching/vomiting, based on patient diary records and assessed by each grade, were comparable and were not significantly different between the two groups. During the PEEP, the frequency of nausea was lower in the GTS group (p = 0.0049) while the frequency of retching/vomiting between the groups was not significantly different, as shown in Supplementary Table 1. For the entire study period, the percentage of patients requiring rescue medication was 19.74% (n = 30) in the GTS group and 16.87% (n = 28) in the ondansetron group (p = 0.5081).
In the assessment of satisfaction with FLIE during the study period, the mean ┬▒ standard deviation VAS score for patients in the GTS group was 1,547.38 ┬▒ 306.00 mm (median, 1,662.11) and 1,494.07 ┬▒ 312.05 mm (median, 1,591.58) in the ondansetron group. The GTS group had a significantly higher score in the question of nausea than the ondansetron group (p = 0.0049, Wilcoxon rank-sum test).
In the GTS group, we evaluated the adhesive properties of the patches in the PPS. Overall, 119 (78.29%) patients had Ōēź 90% adhesion, 26 (17.11%) had Ōēź 75% adhesion, six (3.95%) had Ōēź 50% adhesion, and none of the patients had < 50% adhesion during the entire study period. The patch completely detached in one patient (0.66%).
In total, 381 patients were included in the SS, and the overall incidence of adverse events (AE) excluding nausea and retching/vomiting was 70.87% (133 GTS, 137 ondansetron, p = 0.2418). A greater number of AE was reported in the ondansetron group (316 events) than in the GTS group (277 events). The common AE (incidence rate Ōēź 5%) were constipation 15.75% (n = 60), decreased appetite 14.96% (n = 57), dyspepsia 8.14% (n = 31), hiccups 7.09% (n = 27), and cough 5.25% (n = 20) in both groups. Adverse drug reactions (ADRs) that were reported during the study are listed in Table 3. Constipation was the most common ADR in both treatment groups (5.64% and 4.84% in the GTS and ondansteron group, respectively). Regarding the skin tolerability of the GTS, patch related AE, pruritus 0.51% (n = 1) was reported.
Following the initial U.S. Food and Drug Administration (FDA) approval of GTS in 2008, there have been no reports directly comparing the efficacy and safety of the GTS with other 5-HT3 RAs, in combination with dexamethasone and NK-1 RA, in CINV associated with multiday HEC in solid cancer patients [14]. This study is the first randomized, phase IV trial to compare the efficacy and safety of the GTS with a representative 5-HT3 RA, ondansetron, that was administered IV and orally in Korean cancer patients with HEC.
In the primary efficacy analysis, the GTS was found to be non-inferior to ondansetron for the control of CINV in patients receiving multiday treatment with HEC. Exploratory analyses reveal similar results, indicating that there were no significant differences in secondary endpoints, which further supports the findings of the current study. In per day analysis, the GTS provided effective control of delayed emesis in HEC regimen. On the first day, the CR and CC rates of the ondansetron group were higher, because the effective plasma concentration was attained immediately by IV administration. However, the GTS group had higher CR and CC rates during later days. The CC rate assessing nausea as well as vomiting and rescue medication was significantly higher on the fifth day (p = 0.0278). This result indicates that peak plasma concentration was gradually reached, and steady state concentration was maintained with long-lasting effects against delayed emesis in the GTS group. When we assessed primary and secondary efficacy, PEEP was used in addition to per study day and overall study period. Because transdermal drug delivery systems have the advantage of prolonged efficacy, we considered the assessment of effects on delayed emesis to be important for evaluation of the GTS.
This non-inferiority study design was appropriate for evaluation of the GTS. However, the FDA has recently removed the 32 mg single IV dose of ondansetron from the prescription label due to evidence of prolongation of QT interval. Therefore, the National Comprehensive Cancer Network (NCCN) guideline recommended usual dose of ondansetron used during this study period may not be relevant in current clinical scenarios. The guideline recommended period and dose of ondansetron treatment used during this study was 8 to 16 mg (maximum, 32 mg/day) IV or 16 to 24 mg orally only on day 1 [2]; however, patients in the ondansetron group took ondansetron 8 mg twice daily for 4 more days during the study to be comparable to the GTS, which can release granisetron for up to 7 days. Furthermore, at the request of our IRB, the treatment schedule with ondansetron was extended to match the GTS group. Nevertheless, when we compared the matched doses, the primary endpoint was established, and the efficacy of GTS was maintained for all grades of nausea and retching/vomiting on individual study days, and the CC and CR rates were comparable for both groups. In the pharmacokinetics report, the half-life of ondansetron after 8 mg oral dose was approximately 3 to 4 hours [15], indicating that repetitive ondansetron or rescue drugs may be used to treat emesis during the rest time of the chemotherapy. However, GTS provided similar mean plasma concentration of 2.2 ng/mL on day 1 and 5 [3], which may result in relatively less use of rescue treatment for control of CINV.
There were no significant differences in the safety profile between groups. The lower percentage of AE in the GTS group indicated that the regimen was tolerated by patients receiving multiday HEC. The skin tolerability of the GTS was acceptable with higher satisfaction based on patient diary records (FLIE) and, thus, precludes patient concerns regarding elasticity and localized dermal irritation with the use of the GTS. The incidence of constipation was higher in the GTS group. However, the rate of constipation was similar to that in a previous study [3].
Current clinical guidelines recommend the addition of corticosteroid to 5-HT3 RAs to improve emetic control [2]. Furthermore, the addition of NK-1 RA to 5-HT3 RA plus dexamethasone has demonstrated good control of delayed CINV [16]. Similarly, in our study we demonstrated that the addition of both a standardized dose of NK-1 RA and corticosteroid to GTS therapy during HEC regimens was appropriate for controlling CINV, especially during the later days of chemotherapy. The addition of corticosteroid and NK-1 RA to the GTS is expected to be well tolerated, as other 5-HT3 RAs are commonly used in combination.
Rates of CINV can be decreased with the appropriate use of various antiemetics. However, there are several limitations related to oral and IV administration [3]. In a previous study comparing two different antiemetic treatments in 36 patients receiving high dose cisplatin, patient compliance with multiple oral drugs decreased by an average of 24% after leaving the hospital [17]. In addition, some cancer patients have aversions against oral medications, particularly when patients have experienced previous gastrointestinal effects, undergone head and neck treatments including surgery, radiation, or have comorbidities such as xerostomia and mucositis [18]. Furthermore, IV administration of an antiemetic may not be suitable, particularly for multiday chemotherapy regimens, because of inconvenience to patients or increased use of resources (e.g., additional physician or nursing time, catheter devices, chair time at the chemotherapy clinic) [17,18]. The GTS provides constant blood concentrations and additional advantages of transdermal drug delivery, which include avoiding gastrointestinal irritation and hepatic first-pass metabolism, minimizing the AE associated with peak plasma drug concentrations, and improved convenience and compliance [19]. Such controlled drug delivery with constant drug release through the skin to the systemic circulation is the main benefit of the GTS, in addition to the significant improvement in the QoL on FLIE as compared with ondansetron.
This randomized study suggests that the GTS is an appropriate agent of 5-HT3 RA administration for prevention of CINV in cancer patients receiving multiday HEC with comparable efficacy, safety, improved satisfaction, and convenience to established therapies.
1. Chemotherapy induced nausea and vomiting (CINV) constitutes one of the most unpleasant complications among patients receiving highly emetogenic chemotherapy (HEC).
2. This phase IV andomized controlled trial compared the efficacy and safety of the granisetron transdermal system (GTS) with ondansetron-containing antiemetics in Korean cancer patients with HEC.
3. The GTS is well tolerated and non-inferior to dose- and duration-matched ondansetron for controlling CINV. Patient satisfaction per Functional Living Index-Emesis was significantly higher in the GTS group.
4. The GTS is an appropriate choice of 5-HT3 receptor antagonists administration for prevention of CINV in cancer patients receiving multiday HEC, with comparable efficacy, safety, improved satisfaction, and convenience to established therapies.
Supplementary Information
Supplementary Table 1.
Maximum severity and frequency of nausea and retching/vomiting (per protocol set)
Notes
CRedit authorship contributions
Der Sheng Sun: data curation, formal analysis, methodology, writing - original draft, writing - review & editing; Yoon Ho Ko: data curation, methodology; Jong Youl Jin: data curation, methodology; In Sook Woo: data curation, methodology; Suk Young Park: data curation, methodology; Yun Ae Eom: data curation, formal analysis; Jin Hyoung Kang: conceptualization, data curation, formal analysis, funding acquisition, methodology, project administration, visualization; Hoon Kyo Kim: data curation, methodology
Figure┬Ā1
Disposition of patients. GTS, granisetron transdermal system; SAE, serious adverse event; IP, investigational product.
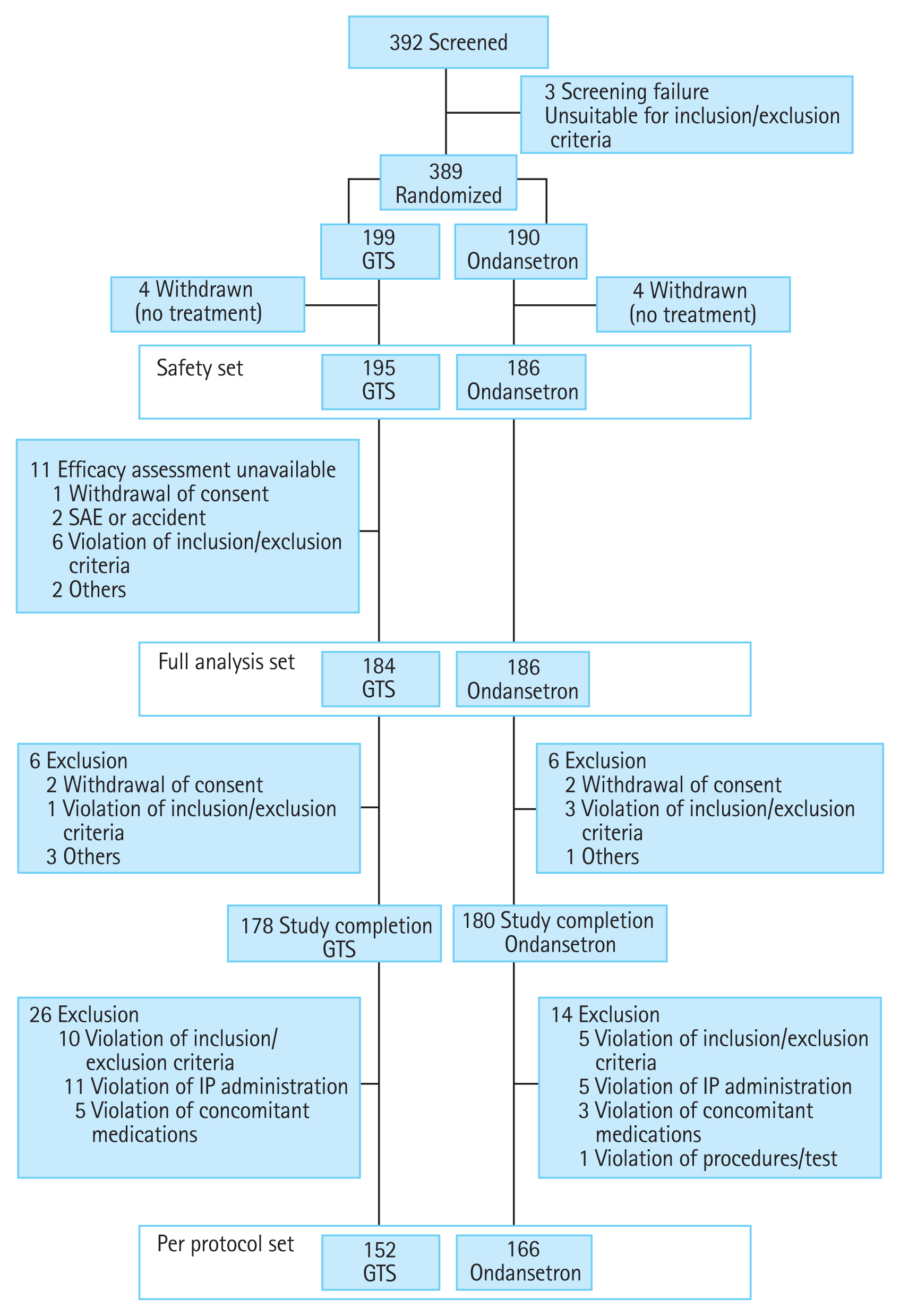
Figure┬Ā2
Percentage of patients who achieved (A) complete response (CR) and (B) complete control (CC) per study day (per protocol set). GTS, granisetron transdermal system. ap = 0.0278 vs. ondansetron on day 5, chi-square test.
Table┬Ā1
Baseline demographic characteristics (full analysis set)
| Characteristic | GTS | Ondansetron | p value |
|---|---|---|---|
| Number | 184a | 186 | |
| Sex | 0.8557b | ||
| ŌĆāMale | 127 (69.02) | 130 (69.89) | |
| ŌĆāFemale | 57 (30.98) | 56 (30.11) | |
| Age, yr | 60.29 ┬▒ 11.30 | 61.37 ┬▒ 10.02 | 0.4568c |
| Age group, yr | 0.3867d | ||
| ŌĆā20ŌĆō29 | 3 (1.63) | 1 (0.54) | |
| ŌĆā30ŌĆō39 | 3 (1.63) | 3 (1.61) | |
| ŌĆā40ŌĆō49 | 26 (14.13) | 15 (8.06) | |
| ŌĆā50ŌĆō59 | 51 (27.72) | 62 (33.33) | |
| ŌĆā60ŌĆō69 | 53 (28.80) | 58 (31.18) | |
| ŌĆāŌēź 70 | 48 (26.09) | 47 (25.27) | |
| Height, cm | 162.72 ┬▒ 8.35 | 161.49 ┬▒ 7.82 | 0.1407c |
| Weight, kg | 59.75 ┬▒ 9.23 | 59.89 ┬▒ 10.21 | 0.9969c |
| Smoking status | 0.5050b | ||
| ŌĆāNever smoked | 69 (37.50) | 69 (37.10) | |
| ŌĆāSmoker | 30 (16.30) | 23 (12.37) | |
| ŌĆāEx-smoker | 85 (46.20) | 94 (50.54) | |
| Alcohol, time/week | 0.0488d | ||
| ŌĆāNone | 161 (87.50) | 176 (94.62) | |
| ŌĆā1ŌĆō2 | 9 (4.89) | 5 (2.69) | |
| ŌĆā3ŌĆō4 | 5 (2.72) | 4 (2.15) | |
| ŌĆā5ŌĆō6 | 3 (1.63) | 1 (0.54) | |
| ŌĆāŌēź 7 | 6 (3.26) | 0 | |
| ECOG performance status | 0.3355b | ||
| ŌĆā0 | 19 (10.33) | 20 (10.75) | |
| ŌĆā1 | 149 (80.98) | 157 (84.41) | |
| ŌĆā2 | 16 (8.70) | 9 (4.84) | |
| Primary tumor site | |||
| ŌĆāLung | 71 (38.59) | 78 (41.94) | |
| ŌĆāGastrointestinal | 59 (32.07) | 51 (27.42) | |
| ŌĆāHead or neck | 9 (4.89) | 15 (8.06) | |
| ŌĆāBreast | 12 (6.52) | 10 (5.38) | |
| ŌĆāOther | 33 (17.93) | 34 (18.28) | |
| Metastatic disease | 0.9777b | ||
| ŌĆāYes | 116 (63.04) | 117 (62.90) | |
| ŌĆāNo | 68 (36.96) | 69 (37.10) | |
| History of radiotherapy | 0.4612b | ||
| ŌĆāYes | 33 (17.93) | 39 (20.97) | |
| ŌĆāNo | 151 (82.07) | 147 (79.03) | |
| History of chemotherapy | 0.6766b | ||
| ŌĆāYes | 95 (51.63) | 92 (49.46) | |
| ŌĆāNo | 89 (48.37) | 94 (50.54) | |
| Chemotherapy duration, day | 0.6757b | ||
| ŌĆā1 | 116 (63.04) | 105 (56.45) | |
| ŌĆā2 | 5 (2.72) | 8 (4.30) | |
| ŌĆā3 | 27 (14.67) | 35 (18.82) | |
| ŌĆā4 | 26 (14.13) | 27 (14.52) | |
| ŌĆā5 | 10 (5.43) | 11 (5.91) | |
| Chemotherapy regimen | 0.8350b | ||
| ŌĆāCisplatin chemotherapy | 147 (79.89) | 150 (80.65) | |
| ŌĆāNon-cisplatin chemotherapy | 37 (20.11) | 36 (19.35) | |
Table┬Ā2
Percentage of patients who achieved a complete response during PEEP
| Variable | GTSa | Ondansetron | GTS vs. Ondansetron |
|---|---|---|---|
| PP set | 152b | 166 | |
| ŌĆāCR | 132 (86.84) | 150 (90.36) | |
| ŌĆā95% CI for the rate of CR | 81.47 to 92.22 | 85.87 to 94.85 | ŌłÆ10.52 to 3.48 |
| FA set | 184 | 186 | |
| ŌĆāCR | 158 (85.87) | 167 (89.78) | |
| ŌĆā95% CI for the rate of CR | 80.84 to 90.90 | 85.43 to 94.14 | ŌłÆ10.57 to 2.74 |
Values are presented as number (%). CR defined as no retching/vomiting and no rescue medication. Non-inferiority margin: ŌłÆ0.15 (ŌłÆ15%).
Table┬Ā3
Adverse drug reactions (safety set)
| System organ class preferred term | GTS (n = 195)a | Ondansetron (n = 186) | ||
|---|---|---|---|---|
|
|
|
|||
| Incidence rateb | No. of events | Incidence rate | No. of events | |
| Gastrointestinal disorders | 13 (6.67) | 14 | 10 (5.38) | 12 |
|
|
||||
| ŌĆāConstipation | 11 (5.64) | 11 | 9 (4.84) | 9 |
|
|
||||
| ŌĆāDyspepsia | 3 (1.54) | 3 | 1 (0.54) | 1 |
|
|
||||
| ŌĆāEpigastric discomfort | 0 | 0 | 2 (1.08) | 2 |
|
|
||||
| Respiratory, thoracic, and mediastinal disorders | 0 | 0 | 2 (1.08) | 2 |
|
|
||||
| ŌĆāHiccups | 0 | 0 | 2 (1.08) | 2 |
|
|
||||
| Investigations | 0 | 0 | 1 (0.54) | 1 |
|
|
||||
| ŌĆāBlood glucose increased | 0 | 0 | 1 (0.54) | 1 |
|
|
||||
| Skin and subcutaneous tissue disorders | 1 (0.51) | 1 | 0 | 0 |
|
|
||||
| ŌĆāPruritus | 1 (0.51) | 1 | 0 | 0 |
|
|
||||
| Vascular disorders | 1 (0.51) | 1 | 1 (0.54) | 1 |
|
|
||||
| ŌĆāHot flush | 1 (0.51) | 1 | 1 (0.54) | 1 |
REFERENCES
1. Jantunen IT, Kataja VV, Muhonen TT. An overview of randomised studies comparing 5-HT3 receptor antagonists to conventional anti-emetics in the prophylaxis of acute chemotherapy-induced vomiting. Eur J Cancer 1997;33:66ŌĆō74.


2. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: antiemesis V.2.2020 [Internet] Plymouth Meeting (PA): National Comprehensive Cancer Network, c2020. [cited 2021 Sep 22]. Available from: http://www.nccn.org
.
3. Howell J, Smeets J, Drenth HJ, Gill D. Pharmacokinetics of a granisetron transdermal system for the treatment of chemotherapy-induced nausea and vomiting. J Oncol Pharm Pract 2009;15:223ŌĆō231.


4. Boccia RV, Gordan LN, Clark G, Howell JD, Grunberg SM. Sancuso Study Group. Efficacy and tolerability of transdermal granisetron for the control of chemotherapy-induced nausea and vomiting associated with moderately and highly emetogenic multi-day chemotherapy: a randomized, double-blind, phase III study. Support Care Cancer 2011;19:1609ŌĆō1617.



5. Kim JE, Hong YS, Lee JL, et al. A randomized study of the efficacy and safety of transdermal granisetron in the control of nausea and vomiting induced by moderately emetogenic chemotherapy in Korean patients. Support Care Cancer 2015;23:1769ŌĆō1777.


6. Seol YM, Kim HJ, Choi YJ, et al. Transdermal granisetron versus palonosetron for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: a multicenter, randomized, open-label, cross-over, active-controlled, and phase IV study. Support Care Cancer 2016;24:945ŌĆō952.


7. Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol 1997;15:103ŌĆō109.


8. Lindley CM, Hirsch JD, OŌĆÖNeill CV, Transau MC, Gilbert CS, Osterhaus JT. Quality of life consequences of chemotherapy-induced emesis. Qual Life Res 1992;1:331ŌĆō340.


9. U.S. Food and Drug Administration. Center for Drug Evaluation and Research, Approval package for: application number 21-372, palonosetron: statistical review [Internet] Silver Spring (MD): FDA Center for Drug Evaluation and Research, 2013. [cited 2021 Sep 22]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21-372_Alox_Statr.pdf
.
10. Gralla RJ, de Wit R, Herrstedt J, et al. Antiemetic efficacy of the neurokinin-1 antagonist, aprepitant, plus a 5HT3 antagonist and a corticosteroid in patients receiving anthracyclines or cyclophosphamide in addition to high-dose cisplatin: analysis of combined data from two phase III randomized clinical trials. Cancer 2005;104:864ŌĆō868.


11. Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin. The Aprepitant Protocol 052 Study Group. J Clin Oncol 2003;21:4112ŌĆō4119.


12. Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 2003;97:3090ŌĆō3098.


13. Schmoll HJ, Aapro MS, Poli-Bigelli S, et al. Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Ann Oncol 2006;17:1000ŌĆō1006.


14. U.S. Food and Drug Administration. Sancuso. Approval information [Internet] Silver Spring (MD): FDA, 2014. [cited 2021 Sep 22]. Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails
.
15. Tyers MB, Bunce KT, Humphrey PP. Pharmacological and anti-emetic properties of ondansetron. Eur J Cancer Clin Oncol 1989;25( Suppl 1):S15ŌĆōS19.

16. Jordan K, Sippel C, Schmoll HJ. Guidelines for antiemetic treatment of chemotherapy-induced nausea and vomiting: past, present, and future recommendations. Oncologist 2007;12:1143ŌĆō1150.


17. Chua DT, Sham JS, Au GK, et al. The antiemetic efficacy of tropisetron plus dexamethasone as compared with conventional metoclopramide-dexamethasone combination in Orientals receiving cisplatin chemotherapy: a randomized crossover trial. Br J Clin Pharmacol 1996;41:403ŌĆō408.



- TOOLS



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



