 |
 |
|
|
|
Abstract
Background/Aims
Hypertension is considered a risk factor in immunoglobulin A nephropathy (IgAN). However, after IgAN diagnosis, the relationship between early blood pressure control and renal prognosis remains unclear. This study aimed to analyze the association between the prognosis of IgAN patients and a controlled status of hypertension within the first year of IgAN diagnosis.
Methods
We retrospectively analyzed 2,945 patients diagnosed with IgAN by renal biopsy. The patients were divided into ‘normal,’ ‘new-onset,’ ‘well-controlled,’ and ‘poorly-controlled’ groups using blood pressure data from two consecutive measurements performed within a year. The Kaplan-Meier survival analysis and Cox proportional-hazards regression model were used to survey the independent association between recovery from hypertension and the risk of IgAN progression. The primary endpoint was IgAN progression defined as the initiation of dialysis or kidney transplantation.
Results
Before IgAN diagnosis, 1,239 patients (42.1%) had been diagnosed with hypertension. In the fully adjusted Cox proportional-hazards models, the risk of IgAN progression increased by approximately 1.7-fold for the prevalence of hypertension. In the subgroup analyses, the ‘well-controlled’ group showed a statistically significant risk of IgAN progression (hazard ratio [HR], 3.19; 95% confidence interval [CI], 1.103 to 9.245; p = 0.032). Moreover, the ‘new-onset’ and ‘poorly-controlled’ groups had an increased risk of IgAN progression compared to the ‘normal’ group (HR, 2.58; 95% CI, 1.016 to 6.545; p = 0.046 and HR, 3.85;95% CI, 1.541 to 9.603; p = 0.004, respectively).
The prevalence of end-stage renal disease (ESRD) in Korea is increasing, with glomerulonephritis accounting for 8% of causes [1]. Immunoglobulin A (IgA) nephropathy, the commonest glomerulonephritis globally, is the most common glomerulonephritis in Korea with a prevalence of approximately 30% [2]. Mesangial deposition of IgA is a histological characteristic of IgA nephropathy (IgAN). This disease has been recognized as an autoimmune disease due to the significant interaction between galactose-deficient IgA1 and circulating anti-glycan antibodies [3]. The 30-year kidney survival rate of IgAN is poor, approximately 50.3% [4]. Thus, many IgAN patients progress to ESRD, which creates a heavy socioeconomic burden [5] and individual health problems, including mortality [6]. Therefore, early intervention with correctable factors in IgAN patients may be beneficial for prognosis and socioeconomics.
Various factors are associated with IgAN prognosis. Hypertension [7,8] is a well-known risk factor for IgAN progression. In addition, it was suggested that the presence of proteinuria [7,8], increased serum creatinine [7,8], smoking [9], hematuria [8,10], elevated serum uric acid [11], hypertriglyceridemia [11], and genetic factors [12,13] were related to IgAN prognosis. In addition, blood pressure control in IgAN remains a major concern because there is no evidence from randomized controlled trials suggesting a target blood pressure for IgAN. Moreover, the evidence grade of target blood pressure in the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines is “not graded” [14].
Although hypertension is known to be related to IgAN prognosis, no studies have been conducted to determine how a controlled hypertension status after IgAN diagnosis affects IgAN prognosis. In this study, we aimed to analyze the effect of a controlled blood pressure on the renal outcome of patients within the first year after IgAN diagnosis.
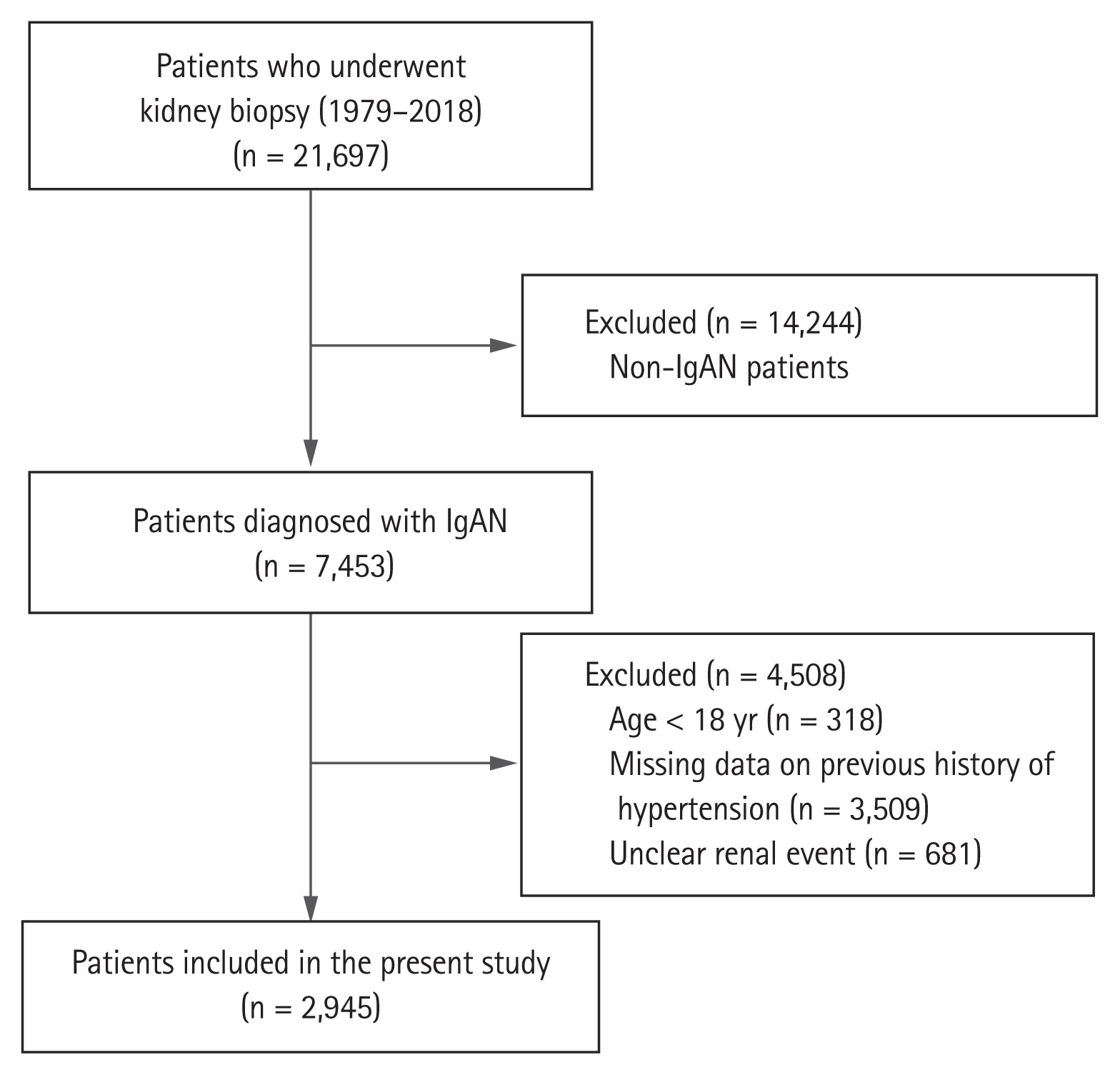
Of 21,697 patients who underwent kidney biopsy between January 1979 and October 2018 at eighteen university hospitals in Korea (Kyungpook National University Hospital, Kyung Hee University Hospital at Gangdong, Hallym University Kangdong Sacred Heart Hospital, Gangnam Severance Hospital, Korea University Guro Hospital, Korea University Anam Hospital, Eulji University Hospital, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul National University Bundang Hospital, Seoul National University Hospital, Severance Hospital, Pusan National University Yangsan Hospital, Eunpyeong St. Mary’s Hospital, Ewha Womans University Mokdong Hospital, National Health Insurance Service Ilsan Hospital, Chonnam National University Hospital, Jeonbuk National University Hospital, and Hallym University Sacred Heart Hospital), 7,453 patients were diagnosed with IgAN. We excluded 318 patients under the age of 18 years and 3,509 patients who had missing data on previous history of hypertension. Further, 681 patients who were unsure about whether they had previously experienced a renal event were excluded; finally, 2,945 patients were analyzed in this study (Fig. 1).
The primary endpoint of this study was IgAN progression, defined as the initiation of dialysis or kidney transplantation. Anemia was defined as hemoglobin level below 13 mg/dL for men and below 12 mg/dL for women [15]. Serum creatinine was measured using traceable isotope dilution mass spectrometry. Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease equation [16]. The entire study population was classified into four subgroups based on a previous history of hypertension and two examinations of blood pressure (at 6 and 12 months) performed during the first year of follow-up. Patients who did not have a history of hypertension and whose systolic and diastolic blood pressures were ≤ 130 and ≤ 80 mmHg, respectively, in both blood pressure examinations were assigned to the ‘normal’ group. Patients who did not have a history of hypertension but whose systolic or diastolic blood pressure was ≥ 130 or ≥ 80 mmHg, respectively, in any of the two blood pressure examinations were assigned to the ‘new-onset’ group. Patients who had a history of hypertension but whose systolic or diastolic blood pressure was ≥ 130 or ≥ 80 mmHg, respectively, in both blood pressure examinations were assigned to the ‘well-controlled’ group. Patients who had a history of hypertension and whose systolic or diastolic blood pressure was ≥ 130 or ≥ 80 mmHg, respectively, in any of the two blood pressure examinations were assigned to the ‘poorly-controlled’ group.
Continuous variables were tested for normality using Shapiro-Wilk test, and normally distributed data are described using means ± standard deviations. Skewed data are expressed using medians and interquartile ranges (IQR). Student’s t test was applied for normally distributed data, and the Mann-Whitney U test was applied for skewed data, to assess differences and clinical characteristics between the control and hypertension groups. Categorical variables are expressed as percentages, and the chi-square test was applied to compare the control and hypertension groups. The Cochran-Armitage trend test was conducted for categorical variables that had more than two categories. With the exception of urine protein/creatinine ratio (UPCR) (13.9%), the percentages of missing data in all variables were less than 10%. We used the multiple imputation method for missing data with the mice package [17] in R because missing values in clinical epidemiological data are generally the missing at random type [18] and Madley-Dowd et al. [19] showed that the multiple imputation method might reduce bias in data which is missing at random. To establish the effect of a controlled status of hypertension on IgAN progression, we applied the Kaplan-Meier survival curve with log-rank test and a univariate Cox proportional-hazards model. Additionally, in adjusted Cox proportional-hazards models, we used covariates that are clinically known to be related to IgAN progression and which showed significant results in the univariate analyses. Test for collinearity was performed to assess the mutual influence between variables. Proportional hazards assumption of the Cox proportional-hazards models was verified by Schoenfeld residuals. Serum albumin violated the proportional hazards assumption, and stratified Cox proportional-hazards model with hypoalbuminemia (no vs. yes) was applied. In the stratified Cox proportional-hazards model analysis, interaction analyses between hypoalbuminemia and hypertension and between hypoalbuminemia and a controlled status of hypertension were performed. Hazard ratio (HR) and 95% confidence interval (CI) were calculated to compare the risk of IgAN progression. Data were analyzed and plotted using R language version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria) [20]. All statistical tests were two-tailed, and p values < 0.05 were considered statistically significant.
This study adhered to the tenets of the Declaration of Helsinki. Because the database used in this study did not include personal identifiers and the study is retrospective and observational in design, the need for informed consent was waived. Ethical approval was received from the Chonnam National University Hospital Institutional Review Board (CNUH-EXP-2020-034).
A total of 2,945 patients were analyzed. The median (IQR) follow-up period was 6.42 years (3.82 to 9.27). The mean ± SD age of all patients was 40.3 ± 13.9 years and 47.5% of patients were men. A total of 207 (7.0%) patients had diabetes mellitus, and the mean ± SD eGFR was 76.1 ± 31.3 mL/min/1.73 m2. At renal biopsy, 1,239 of the 2,945 patients had hypertension. The hypertension and control groups showed statistically significant differences in clinical characteristics, excluding sex, serum albumin, and total cholesterol. Compared to the control group, the hypertension group were older and had a higher proportion of women and a higher prevalence of diabetes mellitus. In the laboratory findings at renal biopsy, the hypertension group showed higher serum uric acid levels, lower hemoglobin levels, higher serum creatinine levels, and more severe proteinuria. We have summarized the differences in clinical characteristics between the control and hypertension groups in Table 1.
IgAN progression occurred in 213 of the 2,945 patients during the follow-up duration. By group, 135 (10.9%) patients in the hypertension group and 78 (4.6%) patients in the control group experienced IgAN progression. The prevalence of hypertension was associated with IgAN progression in the Cox proportional-hazards model (Table 2). In models 2 and 3 of the stratified Cox proportional-hazards model analysis, interaction analyses showed that there were no statistically significant interactions between hypoalbuminemia and hypertension (p for interaction, 0.963 in model 2 and 0.070 in model 3). As with the crude analysis, in the fully adjusted Cox analyses, hypertension was consistently an independent risk factor for IgAN progression (HR, 1.700; 95% CI, 1.174 to 2.461; p = 0.005).
We performed Kaplan-Meier survival analysis with log-rank tests on subgroups of the entire cohort according to a controlled status of hypertension (Fig. 2). ‘poorly-controlled,’ ‘well-controlled,’ ‘new-onset,’ and ‘Normal’ groups showed poor prognosis in descending order. Interaction analyses between hypoalbuminemia and controlled status of hypertension were performed in the stratified Cox proportional-hazards models (models 2 and 3) and no statistical significance was detected (new-onset, p = 0.589; well-controlled, p = 0.555; poorly-controlled, p = 0.140 in model 2 and new-onset, p = 0.634; well-controlled, p = 0.563; poorly-controlled, p = 0.177 in model 3). compared to the ‘normal’ group, in the crude analysis of the Cox proportional-hazards model, the risk of IgAN progression was increased by approximately 2.9 times in the ‘new-onset’ group, 4.1 times in the ‘well-controlled’ group, and 6.5 times in the ‘poorly-controlled’ group (Table 3). In the fully adjusted Cox proportional-hazards model, the ‘new-onset,’ ‘well-controlled,’ and ‘poorly-controlled’ groups showed statistical significance with HR of 2.579 (95% CI, 1.016 to 6.545), 3.193 (95% CI, 1.103 to 9.245), and 3.847 (95% CI, 1.541 to 9.603), respectively. Notably, although hypertension was well controlled in the first year after diagnosis in the ‘well-controlled’ group, the risk of IgAN progression increased, compared to the ‘normal’ group.
Consistent with the literature, this study demonstrates that the prevalence of hypertension is a risk factor for ESRD. In addition, although blood pressure was well controlled during the first year after IgAN diagnosis, hypertension remained an independent risk factor for IgAN progression.
The prevalence of hypertension in IgAN patients has been reported to vary from 31% to 58% [21–24]. In this study, the prevalence of hypertension was found to be 42.1%, which is similar to the values reported in previous studies. Thus, hypertension is a commonly encountered comorbidity in IgAN patients and its management is important because of its association with the prognosis of these patients, including mortality [25] and renal outcome [26]. In the KDIGO guidelines, target blood pressures of < 130/80 mmHg in IgAN patients with proteinuria of < 1 g/day, and < 125/75 mmHg in those with proteinuria of ≥ 1 g/day are recommended [14]. However, the target blood pressure for IgAN patients has not been established because of limited data. The Modification of Diet in Renal Disease study showed inconsistent results in subgroup and interaction analyses [27]. Recently, Zheng et al. [28] reported the benefits of intensive treatment of blood pressure < 130/80 mmHg for IgAN patients with proteinuria of ≥ 1 g/day in the Prevalence, Awareness and Treatment Rates in Chronic Kidney Disease Patients with Hypertension in China (PATRIOTIC) study. Based on the results of these studies, we defined the target blood pressure as < 130/80 mmHg.
Renal damage due to uncomplicated primary hypertension is conceptually divided into two types: benign and malignant [29]. Benign nephrosclerosis is a non-specific vascular lesion observed in a majority of patients with primary hypertension, and hyaline arteriosclerosis develops slowly. In severe hypertension, malignant nephrosclerosis is observed and is characterized by rapid vascular and glomerular damage. The mechanisms by which hypertension induces renal damage include burdening of systolic blood pressure, buffering of the vascular bed of the kidneys, and local tissue susceptibility to barotrauma [30]. Sustained elevated blood pressure dysregulates renal autoregulation and causes a vicious cycle of poorly controlled blood pressure [31]. Eventually, this leads to loss of kidney function through loss of nephrons and increasing glomerular capillary pressure.
Notably, we found that IgAN patients with preexisting hypertension had poor renal outcomes although blood pressure was well controlled in the first year. However, because only a few randomized and controlled trials have analyzed the effects of hypertension control on renal survival, we could support our results with pre-existing evidence to draw conclusions [32]. Although we cannot explain the precise mechanism of this phenomenon, we believe the following factors may be implicated. First, Konishi et al. [33] reported that IgAN patients developed sodium sensitivity before blood pressure increased, and this was associated with histological damage to the kidneys. In normotensive IgAN patients, increased sodium sensitivity results in histological damage to the kidneys which may be associated with a poor renal prognosis. Second, patients in the ‘well-controlled’ group were older than those in the ‘normal’ and ‘new-onset’ groups (mean age, 44 years old vs. 37 years old and 36 years old). Due to data limitations, we were unable to determine the age of the patients who were initially diagnosed with hypertension. It seemed that the older the patient, the more likely it was to have a longer duration of hypertension, which may have been related to the prognosis of the patients. Third, blood pressure may not have been properly treated after the first year in the ‘well-controlled’ group. Fourth, the factors involved in renal prognosis vary, and the effects of intervention on other major risk factors were not analyzed in this study. Fifth, the ‘well-controlled’ group may have been evaluated as a high-risk group by the physicians and may have received more active treatment, which may have biased the results of this study.
Although our study has many strengths, including a large study population and a long-term follow-up period, our analyses have limitations. First, as in all observation studies, we could not evaluate causality between a controlled status of hypertension and IgAN progression. However, observational studies are powerful tools for assessing epidemiologic relationships and we utilized complimentary analytic methods to robustly analyze the association between blood pressure and IgAN progression [34]. Second, since this study is retrospective, unintended bias related to over-/underdiagnosis or misclassification of patients may exist. For example, in the ‘new-onset’ group, we were unable to clearly distinguish between those whose blood pressure was well controlled and those who were not. Third, we could not determine all sources of bias and confounding factors including variability in blood pressure measurement. Finally, we could not analyze the pathologic classification of IgAN and the effect of antihypertensive drugs and could not exclude the secondary IgAN due to data limitations.
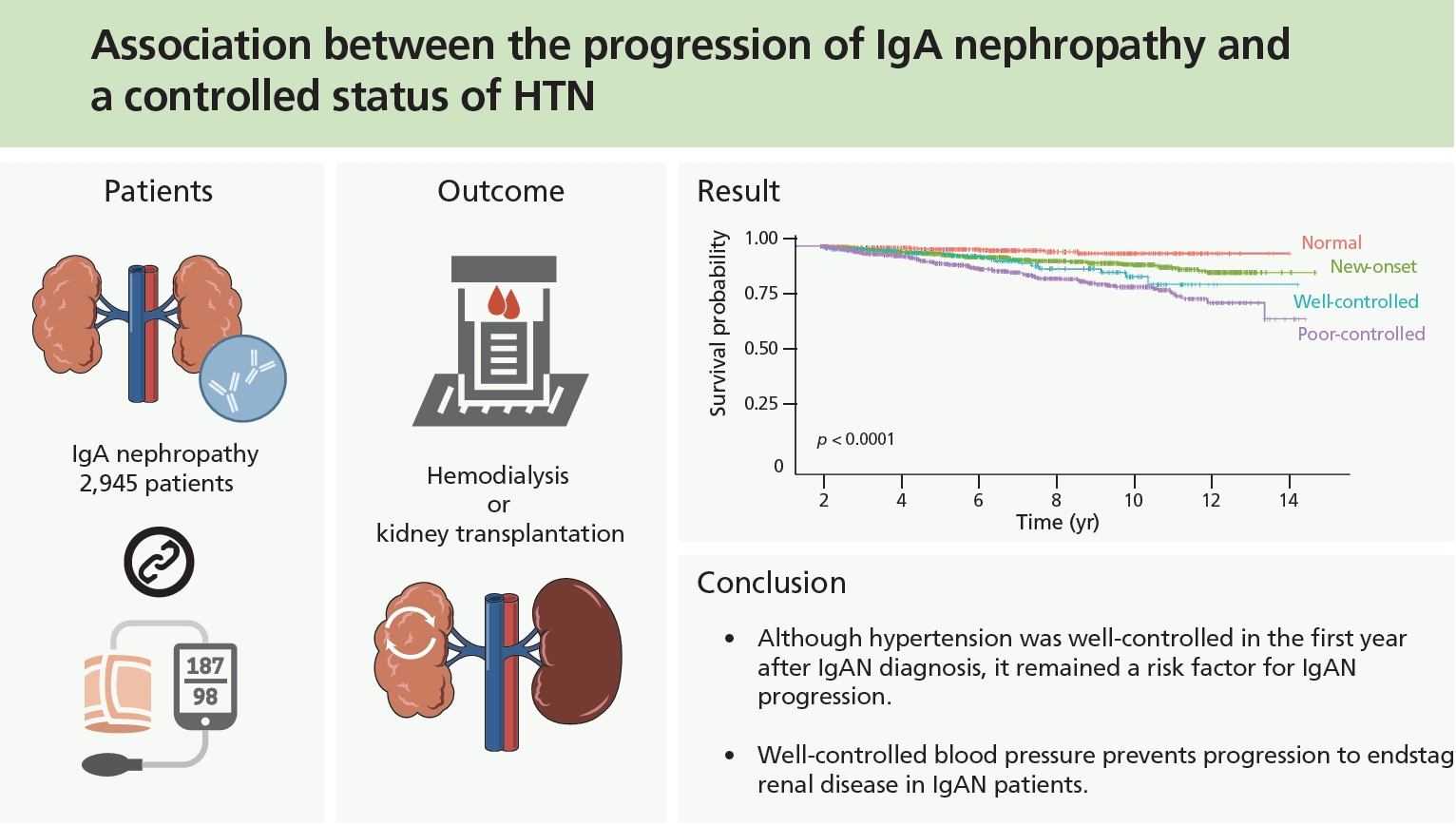
In conclusion, hypertension was a risk factor for the progression of IgAN. Even if blood pressure is well controlled, more careful observation is needed in patients with pre-existing hypertension before IgAN diagnosis, which may be beneficial to patients.
1. Patients with pre-existing hypertension before being diagnosed with immunoglobulin A nephropathy (IgAN) have a poor prognosis when compared to patients without hypertension, even if blood pressure is well controlled later.
2. Well-controlled blood pressure prevents progression to end-stage renal disease in IgAN patients.
Acknowledgments
This work was supported by Cooperative Research Grant 2017 from the Korean Society of Nephrology. The sponsor had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Korean government (MIST) (NRF-2019R1A2C1003971), and Chonnam National University Hospital Biomedical Research Institute Grant (BCRI 20025 & 20076).
Figure 2
Kaplan-Meier survival curve with log-rank test to determine the association between the progression of immunoglobulin A nephropathy and a controlled status of hypertension within the first year.
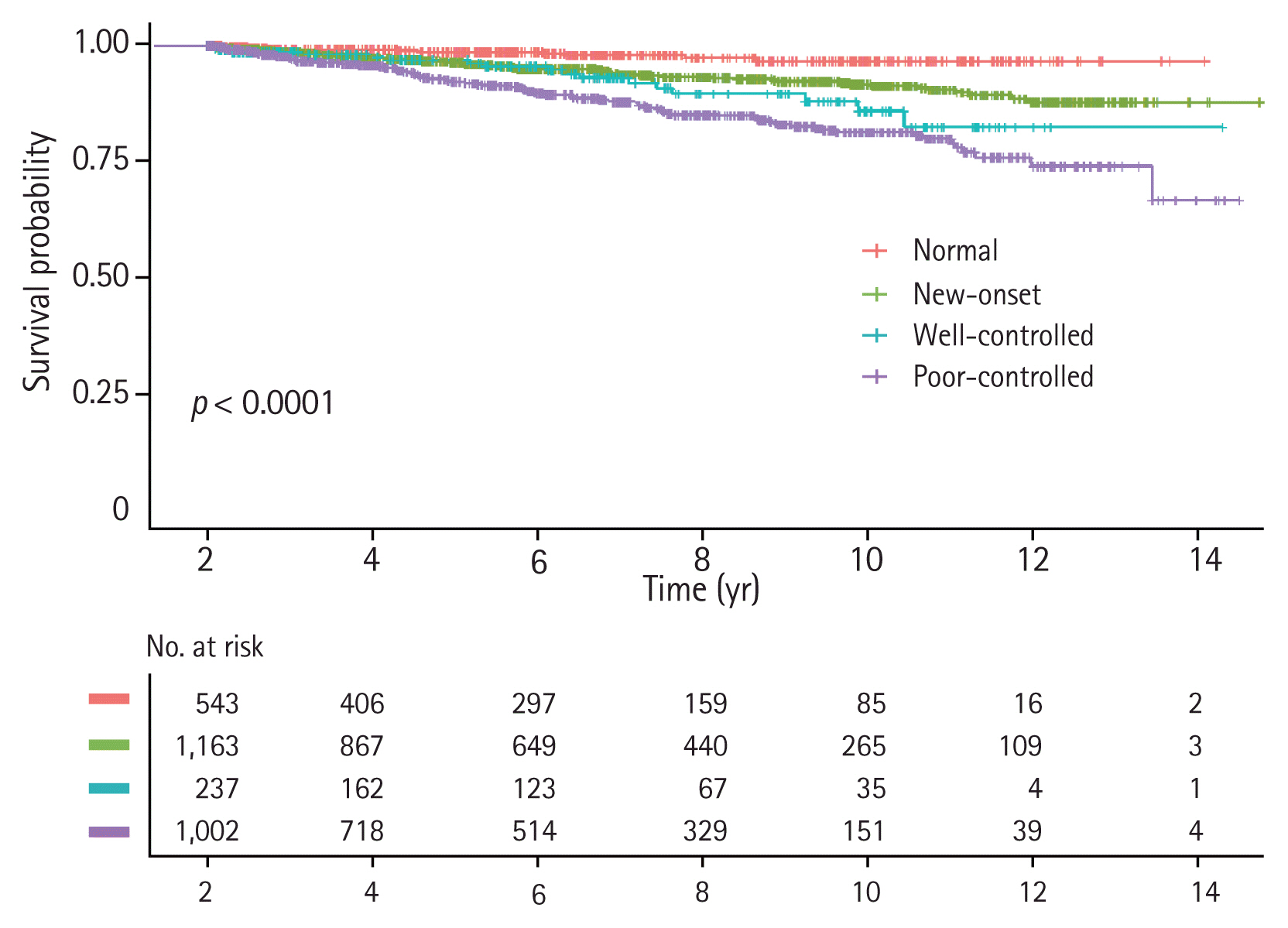
Table 1
Clinical characteristics of the patients using the multiple imputation method
Table 2
Hazard ratio of hypertension for the progression of IgA nephropathy with Cox proportional-hazards models
| Total subject | HR (95% CI) | p value |
|---|---|---|
| Crude | 2.618 (1.980–3.461) | < 0.001 |
| Model 1 | 2.475 (1.844–3.321) | < 0.001 |
| Model 2 | 1.777 (1.229–2.570) | 0.002 |
| Model 3 | 1.700 (1.174–2.461) | 0.005 |
Table 3
Multivariate Cox proportional-hazards models according to a controlled status of hypertension within the first year
REFERENCES
2. Chang JH, Kim DK, Kim HW, et al. Changing prevalence of glomerular diseases in Korean adults: a review of 20 years of experience. Nephrol Dial Transplant 2009;24:2406–2410.


4. Moriyama T, Tanaka K, Iwasaki C, et al. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS One 2014;9:e91756.



5. Hunsicker LG. The consequences and costs of chronic kidney disease before ESRD. J Am Soc Nephrol 2004;15:1363–1364.


6. Kim KM, Oh HJ, Choi HY, Lee H, Ryu DR. Impact of chronic kidney disease on mortality: a nationwide cohort study. Kidney Res Clin Pract 2019;38:382–390.



7. Radford MG Jr, Donadio JV Jr, Bergstralh EJ, Grande JP. Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol 1997;8:199–207.


8. Wakai K, Kawamura T, Endoh M, et al. A scoring system to predict renal outcome in IgA nephropathy: from a nationwide prospective study. Nephrol Dial Transplant 2006;21:2800–2808.


9. Yamamoto R, Nagasawa Y, Shoji T, et al. Cigarette smoking and progression of IgA nephropathy. Am J Kidney Dis 2010;56:313–324.


10. Szeto CC, Lai FM, To KF, et al. The natural history of immunoglobulin a nephropathy among patients with hematuria and minimal proteinuria. Am J Med 2001;110:434–437.


11. Syrjanen J, Mustonen J, Pasternack A. Hypertriglyceridaemia and hyperuricaemia are risk factors for progression of IgA nephropathy. Nephrol Dial Transplant 2000;15:34–42.

12. Kiryluk K, Novak J, Gharavi AG. Pathogenesis of immunoglobulin A nephropathy: recent insight from genetic studies. Annu Rev Med 2013;64:339–356.


13. Kiryluk K, Li Y, Sanna-Cherchi S, et al. Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet 2012;8:e1002765.



14. Cattran DC, Feehally J, Cook HT, et al. Kidney disease: improving global outcomes (KDIGO) glomerulonephritis work group: KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2012;2:139–274.
15. Nutritional anaemias: report of a WHO scientific group. World Health Organ Tech Rep Ser 1968;405:5–37.

16. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–470.


17. Van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw 2010;45:1–67.
18. Pedersen AB, Mikkelsen EM, Cronin-Fenton D, et al. Missing data and multiple imputation in clinical epidemiological research. Clin Epidemiol 2017;9:157–166.



19. Madley-Dowd P, Hughes R, Tilling K, Heron J. The proportion of missing data should not be used to guide decisions on multiple imputation. J Clin Epidemiol 2019;110:63–73.



20. R Core Team. R: A language and environment for statistical computing 2019 [Internet] Vienna (AT): R Foundation for Statistical Computing, 2020. [cited 2021 Mar 19]. Available from: https://www.R-project.org
.
21. Diciolla M, Binetti G, Di Noia T, et al. Patient classification and outcome prediction in IgA nephropathy. Comput Biol Med 2015;66:278–286.


22. Knoop T, Vagane AM, Vikse BE, et al. Addition of eGFR and age improves the prognostic absolute renal risk-model in 1,134 Norwegian patients with IgA nephropathy. Am J Nephrol 2015;41:210–219.


23. Chacko B, John GT, Neelakantan N, et al. Presentation, prognosis and outcome of IgA nephropathy in Indian adults. Nephrology (Carlton) 2005;10:496–503.


24. Le W, Liang S, Hu Y, et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant 2012;27:1479–1485.


25. Berthoux F, Mohey H, Laurent B, Mariat C, Afiani A, Thibaudin L. Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol 2011;22:752–761.



26. Woo KT, Wong KS, Lau YK, Chiang GS, Lim CH. Hypertension in IgA nephropathy. Ann Acad Med Singapore 1988;17:583–588.

27. Upadhyay A, Earley A, Haynes SM, Uhlig K. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med 2011;154:541–548.


28. Zheng Y, Wang Y, Liu S, et al. Potential blood pressure goals in IgA nephropathy: prevalence, awareness, and treatment rates in chronic kidney disease among patients with hypertension in China (PATRIOTIC) Study. Kidney Blood Press Res 2018;43:1786–1795.


29. Olson JL. Hypertension: essential and secondary forms. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, eds. Heptinstall’s Pathology of the Kidney. Philadelphia (PA): Lippincott-Raven, 1998;943–1002.
30. Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension 2004;44:595–601.


31. Bidani AK, Griffin KA. Long-term renal consequences of hypertension for normal and diseased kidneys. Curr Opin Nephrol Hypertens 2002;11:73–80.


32. Praga M, Gutierrez E, Gonzalez E, Morales E, Hernandez E. Treatment of IgA nephropathy with ACE inhibitors: a randomized and controlled trial. J Am Soc Nephrol 2003;14:1578–1583.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



