 |
 |
| Korean J Intern Med > Volume 36(3); 2021 > Article |
|
Abstract
Background/Aims
Postbronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) less than 0.7 using spirometry is the golden standard to diagnose airf low limitation of chronic obstructive pulmonary disease (COPD). Recently, measuring FEV6 has been suggested as an alternative to measure FVC. Studies about the cut-off value for FEV1/FEV6 to diagnose airflow limitation have shown variable results, with values between 0.7 and 0.8. The purpose of this study was to determine the best cut-off value of FEV1/FEV6 to detect airflow limitation using handheld spirometry.
Methods
We recruited subjects over 40 years of age with smoking history over 10 pack-years. Participants underwent measurements with both handheld spirometry and conventional spirometry. We calculated the sensitivity and specificity of the value of FEV1/FEV6 using receiver-operating characteristic (ROC) curve analysis to obtain the diagnostic accuracy of handheld spirometry to detect airflow limitation.
Results
A total of 290 subjects were enrolled. Their mean age and smoking amount were 63.1 years and 31.6 pack-years, respectively. According to our ROC curve analysis, when FEV1/FEV6 ratio was 73%, sensitivity and specificity were the maximum and the area under the ROC curve was 0.93, showing an excellent diagnostic accuracy. Sensitivity, specificity, positive predictive value, and negative predictive value were 86.7%, 89.7%, 88.0%, and 88.5%, respectively. Participants with FEV1/FEV6 Ōēż 73% had lower FEV1 predicted value compared to those with FEV1/FEV6 > 73% (65.4% vs. 86.5%, p < 0.001).
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality. It is predicted to become the fourth leading cause of death worldwide by 2030 [1]. Its prevalence and mortality will increase over time and its direct and indirect socio-economic burden will be extremely high [2].
To treat COPD properly, early diagnosis is important to prevent deterioration of pulmonary function and improve treatment outcomes. Pulmonary function tests using conventional spirometry are required to diagnose airflow limitation of COPD. The ratio of forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) needs to be measured in order to calculate whether postbronchodilator FEV1/FVC is equal to or less than 0.7 as an indicator of airflow limitation [3-5]. However, conventional spirometry is not easy to execute [4,5], because FVC can not be easily measured. In addition, it causes discomfort to patients. Moreover, the result is difficult to reproduce. Thus, COPD is well known to be underdiagnosed in primary care setting [4].
Forced expiratory volume in 6 seconds (FEV6) has been recently suggested as an alternative to FVC to detect airflow limitation [6,7]. In many patients who have difficult with exhalation, measuring FEV6 could shorten expiratory time and reduce patientsŌĆÖ discomfort during examination [8]. Several researches have suggested that cut-off values of FEV1/FEV6 with conventional spirometry are between 0.7 and 0.8 [9,10]. Handheld spirometry can measure both FEV1 and FEV6 simultaneously. Recent active case-finding studies for COPD have shown the effectiveness of handheld spirometry in detecting airflow limitation [9-11]. Therefore, the aim of this study was to find the appropriate cut-off value of FEV1/FEV6 to diagnose COPD using handheld spirometry.
This study was conducted at five tertiary hospitals from April to August 2015. Subjects aged at least 40 years old who had respiratory symptoms and a smoking history of more than 10 pack-years were recruited. We excluded subjects who had a history of disease such as tuberculous sequalae, bronchiectasis, asthma, and lung cancer that might influence pulmonary function tests. Informed written consent was obtained from all participants before participating in this study.
Information such as age, respiratory symptoms (such as cough, sputum, or dyspnea), and smoking history was obtained through history taking. Physical examination was performed by investigators. The degree of dyspnea was evaluated with modified Medical Research Council (mMRC) dyspnea scale. Participants then underwent handheld spirometry without bronchodilator under supervision. The handheld spirometry used in this study was a COPD-6 device (Vitalograph, Buckingham, UK). The test was repeated three times and the highest values of FEV1 and FEV6 were recorded. Detailed procedure for operating the handheld spirometry followed the user manual provided by the manufacture [12]. At the same time, conventional spirometry was performed by well trained technicians according to American Thoracic Society and European Respiratory Society Guideline [13]. Results from conventional spirometry were then compared with those from handheld spirometry.
All data were analyzed using IBM SPSS version 22.0 (IBM Corp., Armonk, NY, USA) and MedCalc version 16.4.3 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org
; 2016). Categorical and continuous data are presented as mean and standard deviation. Variables with non-normal distribution were log-transformed. The relationship between FEV1/FEV6 and FEV1/FVC was evaluated with Pearson correlation analysis. Receiver-operator characteristic (ROC) curves were used to determine a cut-off value for handheld spirometry that had the optimal combination of sensitivity and specificity for diagnosis of airflow limitation defined by a postbronchodilator FEV1/FVC < 0.7. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for the best FEV1/FEV6 value to diagnose COPD. Participants who were diagnosed as COPD by conventional spirometry were divided into two groups according to the newly defined cut-off value of FEV1/FEV6 and characteristics were compared between groups above and below the best cut-off value of FEV1/FEV6 using chi-square test and independent t test. Statistical significance was defined when p value was less than 0.05.
This study was approved by the Institutional Review Board (IRB) of each participating institution. IRB approval number at Hallym University Sacred Heart Hospital was 2015-I027. All subjects provided written informed consent before measurement with handheld spirometry at primary clinic.
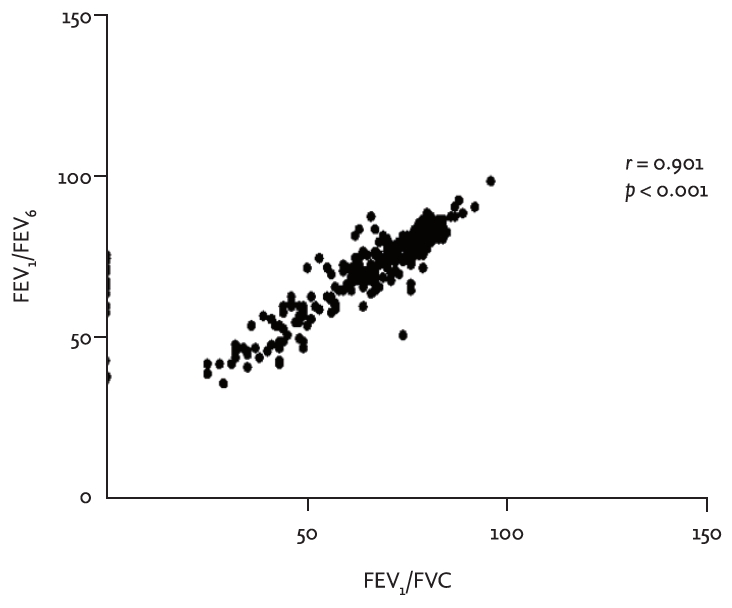
A total of 290 subjects participated in this study. Their mean age was 63.1 years. Their mean smoking history was 31.6 pack-years. Proportions of participants who complained of cough, sputum, and dyspnea were 21.0%, 31.0%, and 39.0%, respectively (Table 1). For conventional spirometry with bronchodilator, mean FEV1 and FEV1/FVC with a bronchodilator were 82.4% ┬▒ 21.1% of the predicted value and 67.5% ┬▒ 14.4%, respectively (Table 1). FEV1/FEV6 for handheld spirometry and postbronchodilator FEV1/FVC for conventional spirometry well correlated with each other (r = 0.901, p < 0.001) (Fig. 1).
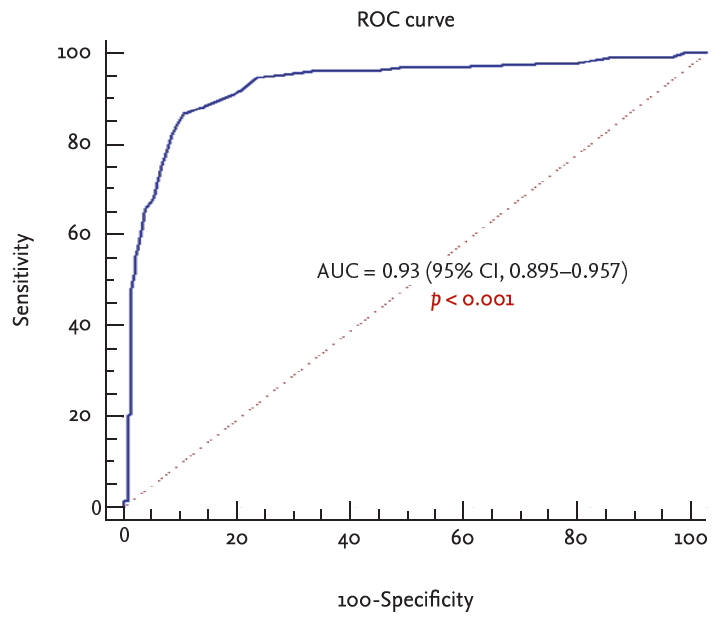
Using postbronchodilator FEV1/FVC < 0.7 as a definition of airflow limitation by conventional spirometry, three values (FEV1/FEV6 Ōēż 73%, FEV1/FEV6 Ōēż 74%, and FEV1/FEV6 Ōēż 77%) were compared against each other. FEV1/FEV6 Ōēż 73% by handheld spirometry had the best sum of sensitivity (86.7%), specificity (89.7%), PPV (88%), and NPV (88.5%) according to the ROC curve analysis for patients diagnosed with COPD. The area under the ROC curve was 0.93 (95% confidence interval [CI], 0.895 to 0.957; p < 0.001) (Table 2, Fig 2).
A total of 139 participants (47.9%) were found to have COPD by conventional spirometry. Among them, 21 patients (15.1%) had FEV1/FEV6 > 73% and 118 (84.9%) had FEV1/FEV6 Ōēż 73% (Table 3). Patients with abnormal FEV1/FEV6 value showed poor lung function significantly in terms of prebronchodilator FEV1, postbronchodilator FEV1, and postbronchodilator FEV1/FVC (Table 3). Differences in age, smoking amount, or respiratory symptoms were not significant between the two groups.
In this study, we tried to find out the best cut-off value of FEV1/FEV6 for diagnosing COPD. With both results of handheld spirometry and conventional spirometry from patients having the risk of COPD, FEV1/FEV6 less than or equal to 0.73 was found to be the best cut-off value to detect airflow limitation. It had high sensitivity and specificity. Furthermore, correlation analysis was performed between FEV1/FEV6 by handheld spirometry and postbronchodilator FEV1/FVC by conventional spirometry. The correlation coefficient was 0.901, showing strong positive correlation between the two.
COPD is a preventable and treatable disease when it is diagnosed and treated early. However, a considerable number of patients might be undiagnosed [14]. One of the reasons is that it is difficult to perform conventional spirometry as a diagnostic tool in primary clinics [4,5]. In Korea, only 19.4% of primary care physicians used spirometry with bronchodilator test to assess COPD patients in 2008 [15]. Unfortunately, this figure did not improve much so far. In recent COPD Clinical Appropriateness Report in Korea, spirometry performance rates in primary clinics were still low, ranging from 37.8% to 42.4% [5]. The main reason for not using the spirometer frequently was the difficulty in performing the test [16]. The obstacle of performing conventional spirometry came from measuring FVC. Thus, FEV6 was suggested as an alternative to FVC. In fact, in previous studies, FEV1/FEV6 and FEV1/FVC showed good agreement, proving that FEV1/FEV6 could be a substitute of FEV1/FVC for detecting airflow limitation. However, these studies were conducted using conventional spirometry [6,7,16].
The most noteworthy aspect of our study was that we analyzed the best value of FEV1/FEV6 using handheld spirometry and that it could detect airflow limitation of COPD as a diagnostic value. There have been studies about the best FEV1/FEV6 value on the use of handheld spirometry to assess subjects at risk for COPD. One cross-sectional study enrolled patients aged 50 years or older who were current smoker or ex-smoker (at least 1 pack-year) and defined the cut-off value to be 0.73 [10]. Another multicenter study recruited patients with high-risk COPD and set the cut-off point for the FEV1/FEV6 ratio < 0.70 with COPD-6 device as prescreening modality of COPD [11]. Although both studies have been conducted to explore active case-finding for COPD with the optimal FEV1/FEV6 value, they used FEV1/FEV6 value obtained from handheld spirometry as a screening purpose [10,11]. On the other hand, our study enrolled participants aged 40 years or older with risk factors for COPD who had respiratory symptoms and more than 10 pack-years of smoking history and found a cut-off value of 0.73 for FEV1/FEV6 with handheld spirometry for diagnosis.
This study has some limitations. First, this study was conducted in a population of patients at risk of COPD, and the cut-off value of FEV1/FEV6 < 0.73 obtained in this study was intended to be a guideline for primary care physicians. In recent COPD Clinical Appropriateness Report in Korea [5], spirometry performance rates in primary care clinics just range from 37.8% to 42.4%. We suggest that the cut-off value we proposed in this study can be helpful in diagnosing and treating COPD patients through COPD-6 device, which is more easily available to primary care physicians who have difficulty in using conventional spirometry. However, even though we considered the value from the previous studies, there was no result from the random population. Therefore, it is also necessary to obtain the validity of our values by collecting nation-wide data in the future. Second, the rate of COPD patients in this study was 47.9%, which was higher than that in other studies [16]. Participants of this study were patients who visited a tertiary hospital directly or were referred from primary clinics with high risk of COPD. Thus, the COPD rate was higher than that in the general population. Third, in our study, of 139 patients diagnosed as COPD with conventional spirometry, approximately 15.1% of patients had FEV1/FEV6 > 0.73 and reported as normal by handheld spirometry. Patients measured with FEV1/FEV6 > 0.73 had higher predicted FEV1 values in both prebronchodilator (84.4% ┬▒ 17.3%) and postbronchodilator (86.5% ┬▒ 17.5%) results. They also had higher postbronchodilator FEV1/FVC than those with FEV1/FEV6 Ōēż 0.73 (86.5 ┬▒ 17.5 vs. 53.8 ┬▒ 11.4, p < 0.001). Patients with FEV1/FEV6 > 0.73 have relatively good lung function. They may not be treated immediately. Additionally, according to updated GOLD guideline [17], the value between 0.6 and 0.8 for postbronchodilator FEV1/FVC might change as a biological variation. Indeed, in a study of two large cohorts [18], diagnostic reversal was observed in some patients with mild to moderate airflow limitation. However, less than 0.6 of postbronchodilator FEV1/FVC at a single measurement would rarely rise to be above 0.7 [17,19]. For these reasons, patients with FEV1/FEV6 > 0.73 should be routinely followed up with handheld spirometry. Fourth, we did not show the gender data. Because there was no gender difference in conducting the study, it was not necessary to distinguish it.
In conclusion, our study indicates that the best cut-off value of FEV1/FEV6 using handheld spirometry to detect airflow limitation would be 73% or less in subjects with COPD risk factors. Further studies are needed to validate this cut-off value.
1. With both results of handheld spirometry and conventional spirometry from patients having the risk of chronic obstructive pulmonary disease, forced expiratory volume in 1 second (FEV1)/forced expiratory volume in 6 seconds (FEV6) less than or equal to 0.73 was found to be the best cut-off value to detect airflow limitation.
2. Furthermore, correlation analysis showed the strong positive correlation between FEV1/FEV6 by handheld spirometry and postbronchodilator FEV1/FVC by conventional spirometry.
Acknowledgments
This study was supported by funds (2014-P33003-00) from the Research of Korea Centers for Disease Control and Prevention.
Figure┬Ā1.
Correlation analysis between forced expiratory volume in 1 second (FEV1)/forced expiratory volume in 6 seconds (FEV6) for handheld spirometry and postbronchodilator FEV1/FVC for conventional spirometry.
Figure┬Ā2.
Receiver-operating characteristic (ROC) curve of forced expiratory volume in 1 second (FEV1)/forced expiratory volume in 6 seconds (FEV6) ratio for diagnosing the postbronchodilator FEV1/FVC < 0.7. AUC, area under the curve; CI, confidence interval.
Table┬Ā1.
Baseline characteristics of study participants (n = 290)
Table┬Ā2.
Sensitivity and specificity of cut-off point of FEV1/FEV6 from spirometry
Table┬Ā3.
Comparison of COPD patients according to the handheld spirometry with cut-off value of FEV1/FEV6 Ōēż 0.73a
| Characteristic | FEV1/FEV6 > 73% (n = 21) | FEV1/FEV6 Ōēż 73% (n = 118) | p value |
|---|---|---|---|
| Age, yr | 68.8 ┬▒ 7.7 | 68.7 ┬▒ 9.4 | 0.971 |
| Smoking amount, pack-year | 34.0 ┬▒ 15.7 | 34.3 ┬▒ 24.7 | 0.964 |
| Presence of respiratory symptom | |||
| ŌĆāCough | 8 (7.3) | 21 (21.7) | 0.737 |
| ŌĆāSputum | 8 (10.8) | 35 (32.3) | 0.242 |
| ŌĆāDyspnea | 16 (16.6) | 50 (49.4) | 0.805 |
| Result of conventional spirometry, % | |||
| ŌĆāPrebronchodilator FEV1 of predicted value | 84.4 ┬▒ 17.3 | 62.6 ┬▒ 18.3 | < 0.001b |
| ŌĆāPrebronchodilator FEV1/FVC | 64.6 ┬▒ 3.6 | 51.8 ┬▒ 11.4 | < 0.001b |
| ŌĆāPostbronchodilator FEV1 of predicted value | 86.5 ┬▒ 17.5 | 65.4 ┬▒ 18.0 | < 0.001b |
| ŌĆāPostbronchodilator FEV1/FVC | 66.2 ┬▒ 3.9 | 53.8 ┬▒ 11.4 | < 0.001b |
REFERENCES
1. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442.



3. Muneswarao J, Verma AK, Hassali MA. Global initiative for chronic obstructive lung disease (GOLD) 2018 report: highlighting an incorrect information. Pulm Pharmacol Ther 2018;49:10.


4. Salinas GD, Williamson JC, Kalhan R, et al. Barriers to adherence to chronic obstructive pulmonary disease guidelines by primary care physicians. Int J Chron Obstruct Pulmon Dis 2011;6:171ŌĆō179.



5. Chung SM, Lee SY. Evaluation of appropriate management of chronic obstructive pulmonary disease in Korea: based on Health Insurance Review and Assessment Service (HIRA) claims. Tuberc Respir Dis (Seoul) 2017;80:241ŌĆō246.



6. Vandevoorde J, Verbanck S, Schuermans D, Kartounian J, Vincken W. FEV1/FEV6 and FEV6 as an alternative for FEV1/FVC and FVC in the spirometric detection of airway obstruction and restriction. Chest 2005;127:1560ŌĆō1564.


7. Swanney MP, Jensen RL, Crichton DA, Beckert LE, Cardno LA, Crapo RO. FEV(6) is an acceptable surrogate for FVC in the spirometric diagnosis of airway obstruction and restriction. Am J Respir Crit Care Med 2000;162(3 Pt 1):917ŌĆō919.


8. Enright RL, Connett JE, Bailey WC. The FEV1/FEV6 predicts lung function decline in adult smokers. Respir Med 2002;96:444ŌĆō449.


9. Kim JK, Lee CM, Park JY, et al. Active case finding strategy for chronic obstructive pulmonary disease with handheld spirometry. Medicine (Baltimore) 2016;95:e5683.



10. Van den Bemt L, Wouters BC, Grootens J, Denis J, Poels PJ, Schermer TR. Diagnostic accuracy of pre-bronchodilator FEV1/FEV6 from microspirometry to detect airflow obstruction in primary care: a randomised cross-sectional study. NPJ Prim Care Respir Med 2014;24:14033.



11. Kjeldgaard P, Lykkegaard J, Spillemose H, Ulrik CS. Multicenter study of the COPD-6 screening device: feasible for early detection of chronic obstructive pulmonary disease in primary care? Int J Chron Obstruct Pulmon Dis 2017;12:2323ŌĆō2331.



12. Vitalograph. VITALOGRAPH 4000 series: respiratory monitors & screening devices [Internet] Lenexa (KS): Vitalograph Inc., 2020. [cited May 27]. Available from: https://vitalograph.com/downloads/view/54
.
13. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011;155:179ŌĆō191.


14. Yoo KH, Kim YS, Sheen SS, et al. Prevalence of chronic obstructive pulmonary disease in Korea: the fourth Korean National Health and Nutrition Examination Survey, 2008. Respirology 2011;16:659ŌĆō665.


15. Park MJ, Choi CW, Kim SJ, et al. Survey of COPD management among the primary care physicians in Korea. Tuberc Respir Dis 2008;64:109ŌĆō124.

16. Chung KS, Jung JY, Park MS, et al. Cut-off value of FEV1/FEV6 as a surrogate for FEV1/FVC for detecting airway obstruction in a Korean population. Int J Chron Obstruct Pulmon Dis 2016;11:1957ŌĆō1963.



17. Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J 2019;53:1900164.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



