 |
 |
| Korean J Intern Med > Volume 33(3); 2018 > Article |
|
Abstract
Background/Aims
Tolvaptan is a very effective treatment for hypervolemic or euvolemic hyponatremia. We compared the clinical efficacy of and response to tolvaptan in patients with the syndrome of inappropriate antidiuretic hormone secretion (SIADH) and congestive heart failure (CHF).
Methods
We retrospectively reviewed the medical records of 50 patients (SIADH, n = 30; CHF, n = 20) who were prescribed tolvaptan between July 2013 and October 2015. Tolvaptan was prescribed when the serum sodium level was < 125 mmol/L and the standard treatment failed. Normonatremia was defined as a serum sodium level of > 135 mmol/L.
Results
After the initiation of tolvaptan therapy, there was an immediate response in the urine volume and serum sodium level in all patients. The improvements in the urine volume and serum sodium concentration were highest within the first 24 hours of treatment. In addition, the mean change in the serum sodium level during the first 24 hours was significantly higher in patients with SIADH than in those with CHF (∆Na, 9.9 ± 4.5 mmol/L vs. 6.9 ± 4.4 mmol/L, respectively; p = 0.025). Also, the mean maintenance dose was lower, and the total duration of tolvaptan use was slightly shorter in the SIADH group than CHF group (21.5 ± 14.9 days vs. 28.0 ± 20.1 days, p = 0.070).
Hyponatremia is a frequent electrolyte disorder in clinical practice [1]. The prevalence of hyponatremia (serum sodium level of < 135 mmol/L) is approximately 2.4%, and severe hyponatremia (serum sodium level of < 125 mmol/L) occurs in about 0.13% of hospitalized patients [2]. Although many patients with hyponatremia are asymptomatic or have mild symptoms, acute severe hyponatremia can cause neurologic problems such as seizures, coma, and respiratory arrest [3,4]. In addition, hyponatremia is associated with an increased risk of mortality, morbidity, and hospitalization as well as increased medical costs [5-7].
Conventional methods for the treatment of patients with euvolemic hyponatremia or hypervolemic hyponatremia are fluid restriction and hypertonic saline infusion using diuretics [4]. However, there are several limitations to these treatments. For example, the efficacy of fluid restriction is limited, and patient compliance is poor. In addition, infusing hypertonic saline may correct sodium levels too rapidly, and diuretics could cause acute kidney injury and electrolyte imbalance [8].
Tolvaptan is an oral selective arginine vasopressin (AVP) V2 receptor antagonist [9]. It blocks the V2 receptor to inhibit the reabsorption of free water through aquaporin channels in the collecting duct, which results in the excretion of electrolyte-free water without electrolyte depletion [9,10]. Tolvaptan was approved for the treatment of hyponatremia by the US Food and Drug Administration in 2008 and the European Medicines Agency in 2009 [11]. The recommended initial dose of tolvaptan is 15 mg once per day; the dose can be increased to 30 mg after 24 hours and to a maximum of 60 mg [10].
However, few studies have assessed the adequate dose of tolvaptan in Korean patients, and no reports have compared the use of tolvaptan in subjects with hypervolemic hyponatremia and euvolemic hyponatremia. There are differences in mechanism of hyponatremia between hypervolemic hyponatremia associated with congestive heart failure (CHF) and euvolemic hyponatremia associated with the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Therefore, we hypothesized that the response of tolvaptan would be different between patients with CHF and SIADH. The aim of this study was to compare the clinical efficacy of tolvaptan for the treatment of hypervolemic hyponatremia associated with CHF and euvolemic hyponatremia associated with SIADH and establish proper use guidelines for tolvaptan use in Korean patients.
We retrospectively reviewed the records of patients who were prescribed tolvaptan between July 2013 and October 2015. All included patients required hospitalization for the treatment of hyponatremia or their primary disease, and serum sodium concentrations were monitored during treatment. Among the 59 eligible patients identified, nine were excluded (four had liver cirrhosis and five had no follow-up laboratory data). The remaining 50 patients were divided into two groups: those with hypervolemic hyponatremia caused by CHF, and those with euvolemic hyponatremia due to SIADH. Hyponatremia was defined as a serum sodium concentration of < 135 mmol/L, and tolvaptan was prescribed when the serum sodium level decreased to < 125 mmol/L and standard treatment failed.
The causes of CHF were ischemic heart disease (70%), valvular heart disease (10%), dilated cardiomyopathy (10%), and stress cardiomyopathy (10%). CHF was confirmed in all patients using echocardiography. SIADH was diagnosed by measuring serum and urine osmolality and the sodium concentration. To exclude other causes of hyponatremia, all patients underwent tests to assess thyroid function and potential adrenal insufficiency.
Data were extracted from the patients’ records, including the serum sodium concentration, serum osmolality, urine sodium, urine osmolality, and urine volume at the time of tolvaptan initiation, after 24 hours, after 48 hours, and at the end of treatment. The primary outcome of this study was to compare the achievement of normonatremia (serum sodium level of > 135 mmol/L) between the two groups. The secondary outcomes were (1) the time required to achieve at least serum sodium level of 130 mmol/L, (2) maintenance dose of tolvaptan, and (3) total duration of tolvaptan use. Any alternative treatments for hyponatremia were stopped during tolvaptan treatment. However, conventional treatments for heart failure, including diuretics and β-blockers, were allowed. The medical ethics committee of the hospital approved the study protocol. For this retrospective chart review which met the regulatory criteria, the informed consent was waived.
Statistical analyses were performed using SPSS version 25.0 (IBM Co., Armonk, NY, USA). All data are expressed as mean ± standard deviation (SD) or median and range. Differences between groups were analyzed using independent t tests for continuous parameters and the Mann-Whitney U test for continuous nonparametric data. Statistical significance was accepted when p < 0.05.
Data from 50 patients (30 with SIADH and 20 with CHF) were analyzed. The mean patient age was 72.1 ± 13.4 years, and 60% were male. The mean baseline serum sodium concentration was 121.5 ± 4.1 mmol/L; there was no difference between the two groups. As expected, the serum uric acid levels were significantly lower in patients with SIADH, whereas renal function was lower in patients with CHF. Patients with SIADH had a slightly higher baseline urine volume than did patients with CHF (Table 1).
There were immediate responses in the urine volume and serum sodium level in all patients after the initiation of tolvaptan therapy. The urine volume immediately increased, and the urine osmolality significantly decreased. As a result, the serum sodium level also increased rapidly in both groups (Table 2). The changes in urine volume and serum sodium concentrations were most prominent in the first 24 hours. The mean change in the serum sodium level in the first 24 hours was higher in patients with SIADH than CHF (ΔNa, 9.9 ± 4.5 mmol/L vs. 6.9 ± 4.4 mmol/L, respectively; p = 0.025) (Fig. 1).
As shown in Table 3, 14 patients with SIADH (46.7%) received an initial tolvaptan dose of 7.5 mg, and the other 16 received 15.0 mg. The dose of tolvaptan was increased in two patients because of insufficient efficacy: one dose was increased from 7.5 to 15.0 mg, and the other was increased from 15.0 to 22.5 mg. The dose of tolvaptan was reduced in six patients with SIADH because the response was too rapid: the dose was reduced from 7.5 to 3.25 mg in two patients and from 15.0 to 7.5 mg in four patients.
In patients with CHF, the initial dose of tolvaptan was 7.5 mg in seven individuals (35%) and 15.0 mg in 13 (65%). The dose was increased in six patients because of an insufficient response: from 7.5 to 15.0 mg in three individuals and from 15.0 to 22.5 and 30.0 mg in three patients. No patients experienced a dose reduction. Therefore, the maintenance dose was significantly higher in patients with CHF than SIADH (p = 0.028).
No patients in the SIADH group failed to achieve normonatremia. However, one patient failed to achieve normonatremia in the CHF group, and this patient expired because of CHF aggravation.
The median time required to achieve a serum sodium level of 130 mmol/L was 1 and 2 days in the SIADH and CHF groups, respectively (p = 0.015). The time taken to achieve a serum sodium level of 135 mmol/L was comparable in both groups (Table 3).
The mean duration of tolvaptan use during hospitalization was 7.90 ± 6.84 and 10.70 ± 6.80 days in the SIADH and CHF groups, respectively (p = 0.366). The total duration of tolvaptan use was slightly longer in patients with CHF than SIADH (28.0 ± 20.1 days vs. 21.5 ± 14.9 days, respectively; p = 0.070). Although the discontinuation of tolvaptan therapy was at the discretion of each patient’s physician, patients with CHF seemed to use tolvaptan for a longer period of time.
In this study, we compared the response of patients with SIADH and CHF to tolvaptan and made several important observations. First, definite improvements in the serum sodium levels occurred in both groups, and tolvaptan was useful in the correction of hyponatremia in both groups. However, the mean change in serum sodium during the first 24 hours was greater in patients with SIADH than CHF. In addition, the median time taken to achieve a serum sodium level of > 130 mmol/L was significantly shorter in the SIADH than CHF group. Also, the mean maintenance dose was smaller and the total duration of tolvaptan use was slightly shorter in the CHF than SIADH group.
In the present study, tolvaptan was effective for the treatment of hyponatremia in all patients with SIADH or CHF. However, the response to tolvaptan treatment differed significantly between the two groups. The early response during the first 24 hours of treatment was better in the SIADH group, and the total duration of tolvaptan use was therefore slightly shorter. In addition, the decrease of urine osmolarity was more definite in SIAHD group than CHF group (–166.3 mOsmol/kg vs. –103.5 mOsmol/kg) during the first 24 hours after the start of tolvaptan. This may explain more rapid correction of serum sodium in SIADH group. Consistent with this finding, recently, some reports showed that urine osmolality before and after the administration of tolvaptan may be an effective predictor of response to tolvaptan in decompensated heart failure patients [12]. Moreover, the mean maintenance dose was lower in the SIADH group. The most common maintenance dose was 7.5 mg (50%) and 15.0 mg (65%) in patients with SIADH and CHF, respectively. Therefore, different tolvaptan treatment strategies should be used in patients with SIADH and CHF.
The reason why there were some difference in response to tolvaptan is considered as follows: hyponatremia is closely related to abnormal water retention caused by increased plasma AVP. AVP secretion is stimulated by the increase in the plasma osmolality via osmoreceptors or decrease in effective blood flow via baroreceptors. In patients with SIADH, AVP secretion is not fully suppressed even in hypo-osmolality, and lead to hyponatremia. In patients with CHF, on the other hand, decreased effective blood flow lead to non-osmotic AVP secretion, which results in hyponatremia [4,13]. We thought that the differences in the mechanism of hyponatremia between the two groups might cause the differences in the response of tolvaptan. In patients with SIADH, tolvaptan is to act independently as a selective V2 receptor antagonist, and hyponatremia is simply corrected by increasing the free water clearance. In patients with CHF, on the other hand, decreased effective blood flow leads to activation of the renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous system as well as non-osmotic AVP release [4]. The RAAS and sympathetic nervous system result in impaired maximum water excretory capacity by decreasing delivery of solute to the diluting site and play a role to retention of water [13]. Tolvaptan is a selective V2 receptor antagonist. The activation of V1a receptor might be able to make differences in the response of tolvaptan in patients with CHF.
Tolvaptan was well-tolerated in the current study; the most common side effects were thirst and dry mouth. No serious side effects such as severe changes in vital signs or neurologic symptoms were observed, consistent with previous studies [11,14]. Even if the symptoms of hyponatremia are not severe, appropriate treatment is important because hyponatremia is closely associated with mortality [5,15]. Several studies have assessed the efficacy of tolvaptan for the treatment of hypervolemic or euvolemic hyponatremia [11,16]. However, no clinical trials have assessed the appropriate dose of tolvaptan or compared the use of tolvaptan in patients with hypervolemic hyponatremia and euvolemic hyponatremia. The recommended initial dose of tolvaptan is 15 mg once a day in hyponatremic patients with SIADH or CHF [9].
The current study has several shortcomings that must be acknowledged. First, although statistically significant differences were observed between the treatment responses of patients with SIADH and CHF, a relatively few patients were included in the study. As a result, it is difficult to determine what treatment strategy is most appropriate in each patient group. Nevertheless, we believe that this study will help establish the appropriate dose titration and dosing period for tolvaptan in patients with SIADH and CHF. Second, there were some differences in the baseline renal function between the two groups. Glomerular filtration rate (GFR) of all 30 patients with SIADH was more than 50 mL/min/1.73 m2. But epidermal growth factor receptor (eGFR) was less than 50 mL/min/1.73 m2 in 10 of 20 patients with CHF. When the 20 patients with CHF were classified into two groups according to eGFR of 50 mL/min/1.73 m2, there was no significant difference in the maintenance dose and median time to achieve a serum sodium level of > 130 mmol/L. In addition, the increment of mean urine output in 24 and 48 hours were greater in CHF than SIADH group, despite of relatively poor renal function. Indeed, in general, it is known that no difference was found in response to tolvaptan between patients with renal creatinine clearance rates above 10 mL/min and with normal renal function [17,18]. However, because most patients (90%) included in our study had GFR more than 35 mL/min/1.73 m2, there could have been an underestimate the differences in response of tolvaptan. Further large scale studies are needed to stratify the response of tolvaptan according to renal function. Third, we did not stop medications such as spironolactone and digoxin for the treatment of underlying heart failure, which might affect electrolyte imbalance and result in drug interactions [19,20]. Finally, we did not perform long-term follow-up. Therefore, further studies are needed to determine the proper maintenance period after tolvaptan treatment in these patient populations.
In conclusion, tolvaptan treatment was faster and more effective than conventional methods, but the response differed in patients with SIADH and CHF. The early response to tolvaptan treatment was better in patients with SIADH than in those with CHF. In addition, a higher mean maintenance dose of tolvaptan was required in patients with CHF than SIADH. Therefore, different tolvaptan treatment strategies should be used in patients with SIADH and CHF. Further large-scale studies are required to establish the appropriate dose titration and dosing period in patients with hypervolemic and euvolemic hyponatremia.
1. The early response to tolvaptan treatment was better in patients with syndrome of inappropriate antidiuretic hormone secretion (SIADH) than in those with congestive heart failure (CHF).
2. The mean maintenance dose was larger and the total duration of tolvaptan use was slightly longer in the CHF than SIADH group.
3. The tolvaptan treatment strategy should be differed between patients with SIADH and those with CHF
Figure 1.
Comparison of the mean serum sodium level between patients with syndrome of inappropriate antidiuretic hormone secretion (SIADH) and heart failure (HF). The mean change in the serum sodium level in the first 24 hours was significantly higher in patients with SIADH than congestive HF.
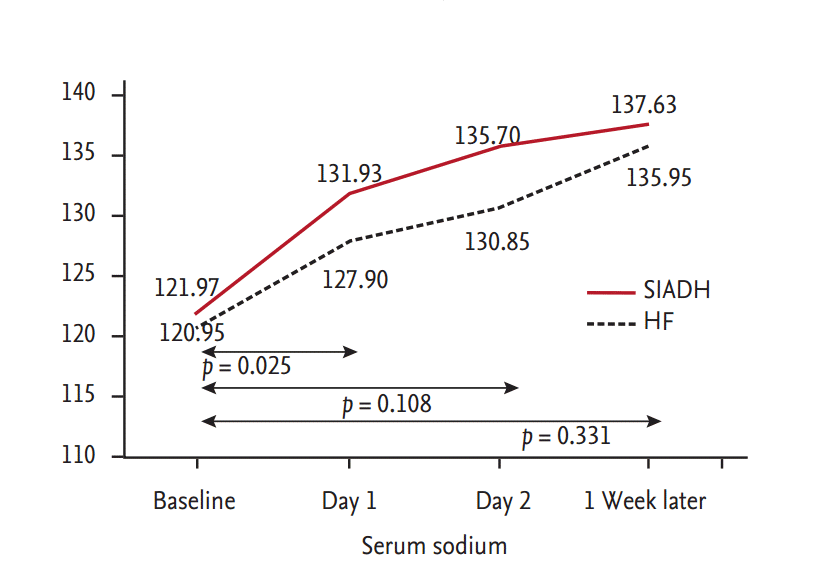
Table 1.
Baseline characteristics of study subjects
Table 2.
Mean laboratory difference between SIADH and CHF during the use of tolvaptan
Table 3.
Comparison of treatment pattern between SIADH and CHF
| Variable | SIADH (n = 30, 66.7%) | CHF (n = 20, 33.3%) | p value |
|---|---|---|---|
| Dose, mg | |||
| Initial | 0.300 | ||
| 7.5 | 14 (46.7) | 7 (35.0) | |
| 15 | 16 (53.3) | 13 (65.0) | |
| Maintenance | 0.028 | ||
| 3.75 | 2 (6.7) | 0 | |
| 7.5 | 15 (50.0) | 4 (20.0) | |
| 15 | 12 (40.0) | 13 (65.0) | |
| 22.5 | 1 (3.3) | 1 (5.0) | |
| 30 | 0 | 2 (10.0) | |
| Time, daya | |||
| Until Na 135 mmol/L | 2 | 4 | 0.663 |
| Until Na 133 mmol/L | 1.5 | 2 | 0.104 |
| Until Na 130 mmol/L | 1 | 2 | 0.015 |
| Achievement of normonatremia | 30 (100.0) | 19 (95.0) | - |
| Tolvaptan use during hospitalization, day | 7.9 ± 6.8 | 10.7 ± 6.8 | 0.366 |
| Total tolvaptan use, day | 21.5 ± 14.9 | 28.0 ± 20.1 | 0.070 |
REFERENCES
1. Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med 2006;119(7 Suppl 1):S30–S35.


2. Kim H, Lee H, Park HC, et al. Characteristics of severe hyponatremia in hospitalized patients: a comparison according to the presence of liver disease. Korean J Nephrol 2008;27:678–687.
3. Gross P, Reimann D, Henschkowski J, Damian M. Treatment of severe hyponatremia: conventional and novel aspects. J Am Soc Nephrol 2001;12 Suppl 17:S10–S14.


4. Verbalis JG, Goldsmith SR, Greenberg A, Schrier RW, Sterns RH. Hyponatremia treatment guidelines 2007: expert panel recommendations. Am J Med 2007;120(11 Suppl 1):S1–S21.


5. Gheorghiade M, Rossi JS, Cotts W, et al. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE trial. Arch Intern Med 2007;167:1998–2005.


6. Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med 2009;122:857–865.



7. Zilberberg MD, Exuzides A, Spalding J, et al. Epidemiology, clinical and economic outcomes of admission hyponatremia among hospitalized patients. Curr Med Res Opin 2008;24:1601–1608.


8. Peri A. Clinical review: the use of vaptans in clinical endocrinology. J Clin Endocrinol Metab 2013;98:1321–1332.



9. Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 2006;355:2099–2112.


10. Nemerovski C, Hutchinson DJ. Treatment of hypervolemic or euvolemic hyponatremia associated with heart failure, cirrhosis, or the syndrome of inappropriate antidiuretic hormone with tolvaptan: a clinical review. Clin Ther 2010;32:1015–1032.


11. Verbalis JG, Adler S, Schrier RW, et al. Efficacy and safety of oral tolvaptan therapy in patients with the syndrome of inappropriate antidiuretic hormone secretion. Eur J Endocrinol 2011;164:725–732.



12. Imamura T, Kinugawa K, Shiga T, et al. Novel criteria of urine osmolality effectively predict response to tolvaptan in decompensated heart failure patients: association between non-responders and chronic kidney disease. Circ J 2013;77:397–404.


13. Narayen G, Mandal SN. Vasopressin receptor antagonists and their role in clinical medicine. Indian J Endocrinol Metab 2012;16:183–191.



14. Berl T, Quittnat-Pelletier F, Verbalis JG, et al. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol 2010;21:705–712.



15. Klein L, O’Connor CM, Leimberger JD, et al. Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study. Circulation 2005;111:2454–2460.


16. Konstam MA, Gheorghiade M, Burnett JC Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial. JAMA 2007;297:1319–1331.


17. Gheorghiade M, Niazi I, Ouyang J, et al. Vasopressin V2-receptor blockade with tolvaptan in patients with chronic heart failure: results from a double-blind, randomized trial. Circulation 2003;107:2690–2696.


18. Garcha AS, Khanna A. Review of tolvaptan in the treatment of hyponatremia. Clin Med Insights Ther 2011;3:315–325.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



