INTRODUCTION
Primary adenocarcinoma of the bladder is a malignant neoplasm derived from urothelium of the bladder showing histologically pure glandular differentiation [
1]. Primary adenocarcinoma of the bladder is a rare malignancy, representing only 0.5% to 2.5% of all malignant bladder neoplasm [
2,
3]. This tumor is more common in males with approximate mean age of 50 to 60 years [
4,
5]. Generally, the tumor is very aggressive, with metastatic disease reported in up to 25% to 40% of patients at the time of diagnosis [
6,
7]. The pathogenesis of primary adenocarcinoma of the urinary bladder has not been fully elucidated. Intestinal metaplasia has been speculated to be a precursor lesion [
8], largely because it is often seen in mucosa adjacent to primary adenocarcinoma of the urinary bladder. The diagnosis of a primary adenocarcinoma of the bladder should only be made when the carcinoma exhibits pure glandular differentiation. In many cases of adenocarcinoma of the bladder, the most important and the most difficult task is to differentiate these tumors from metastatic adenocarcinoma from other organs such as colon, lung, prostate, breast, and uterus. The most frequent secondary tumor involving the urinary bladder is adenocarcinoma of the colon, which morphologically resembles adenocarcinoma of the urinary bladder (enteric type). Because of its rarity, role of chemotherapy for advanced bladder adenocarcinoma is still questionable. Therefore, we performed a retrospective analysis of the clinical features and chemotherapy outcomes of advanced bladder adenocarcinoma to evaluate the clinical findings at presentation, treatment modality and outcome, overall survival (OS) and progression-free survival (PFS) and prognostic factors.
METHODS
Patients
We retrospectively reviewed the patients diagnosed with bladder adenocarcinoma at 10 Korean medical centers from 2005 to 2014. All the patients diagnosed adenocarcinoma through the tissue biopsy, and should have been treated at least one cycle of palliative chemotherapy for metastatic lesion. Adjuvant treatment after operation was excluded. And the patients who had favored primary colon, lung, or ovary lesion were excluded from the study.
Unified case report forms were provided to participating institutions. The collected data included age, gender, performance status, presenting symptom, stage, location of metastatic lesions, hemoglobin, initial date of diagnosis, and chemotherapy regimen utilized. We also obtained data regarding time to relapse, relationship with primary site and relapsing site, salvage treatment modality, and response and survival rates of salvage treatment. Responses were classified according to the Response Evaluation Criteria in Solid Tumors criteria. This study was approved by the Institutional Review Board of Dong-A University Hospital (DAUHIRB-14-108).
Statistical analysis
PFS was estimated from the date treatment began to the date when disease progression was recognized, or the date of the last follow-up visit, or the date of death. OS was calculated from the first day of diagnosis with metastatic or recurrent disease to the date of death or the last follow-up evaluation. OS and PFS were estimated using the Kaplan-Meier product-limit method. Survival rates were compared for statistical differences using log-rank analysis. The Cox regression model was used for multivariate analysis with factors that had been used in univariate (log rank) analysis of OS and PFS. All statistical analyses were performed using the IBM SPSS version 20.0 (IBM Co., Armonk, NY, USA). p value less than 0.05 was considered statistically significant and all p values corresponded to two-sided significance tests.
DISCUSSION
Our study evaluated the efficacy of palliative chemotherapy for adenocarcinoma of bladder and until date, it is one of the largest reports on the efficacy of palliative chemotherapy for the treatment of advanced bladder adenocarcinoma, even though the number of patients was relatively small and retrospective analysis.
Usually it has been reported that male is predominant in bladder adenocarcinoma [
4,
5,
9]. Because of small study population, numbers of female was a little bite larger than male in this study. Nontransitional cell urothelial carcinoma including adenocarcinoma, squamous cell carcinoma, or small cell carcinoma is an uncommon tumor and accounts for only 5% to 10% of urothelial cancers [
10,
11]. Thus, the clinical experience with chemotherapy is limited, and to date, treatment recommendations have largely been derived based on case reports and small series [
11-
14]. Galsky et al. [
12] reported the chemotherapeutic outcomes for 20 patients with nontransitional cell carcinoma of the urothelial tract (11 patients with adenocarcinomas, eight patients with squamous cell carcinomas, and one patient with small cell carcinoma), using a chemotherapeutic regimen consisting of palitaxel, cisplatin, and ifosfamide with mesna. In the reported study, seven patients had a CR or PR, with an objective response rate of 35% (36% in adenocarcinoma). The median OS was 24.8 months in patients with adenocarcinomas and 9 months in those with squamous cell carcinomas. A study by Hong et al. [
4] showed an objective response rate of 36% to various first-line chemotherapeutic regimens and a median OS of 47 months in 14 patients with adenocarcinoma. Siefker-Radtke [
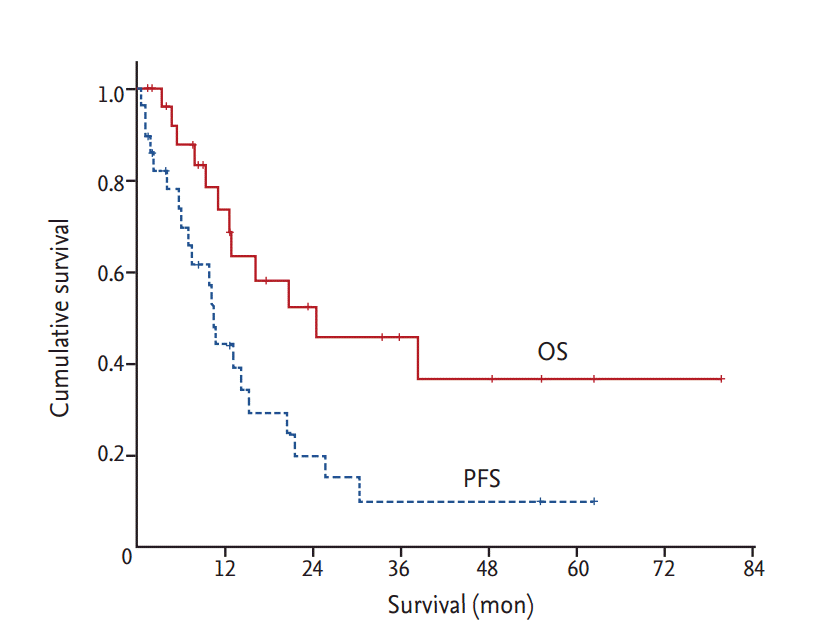
13] reported median survival from recognition of metastatic disease as 24 months in 26 patients in whom metastases ultimately developed. Chemotherapy with various regimens for metastatic disease produced only four significant responses, including three of nine patients treated with 5-FU and cisplatin containing regimens. As per our result, 44.8 % of overall response rate was higher and 24.5 months of median survival was comparable with prior studies (
Table 5).
When we choose the chemotherapeutic regimen, we donŌĆÖt have any evidence based consensus regimen for bladder adenocarcinoma. Transitional cell cancers (TCCs) are treated with gemcitabine-platinum combination, MVAC, or MVP regimens, while adenocarcinoma is usually treated with 5-FU based combination chemotherapy. In this study, TCC focused treatment and adenocarcinoma focused treatments were effective and no differences in PFS and OS. Considering an objective response rate of 39% to 65% to the MVAC or gemcitabine-platinum regimens in advanced transitional cell carcinoma [
15-
17], the objective response of palliative chemotherapy in advanced bladder adenocarcinoma is comparatively lower than transitional cell carcinoma. However, the OS of 24.5 months of this study showed comparable survival results with previous studies regarding transitional cell carcinoma patients [
18,
19].
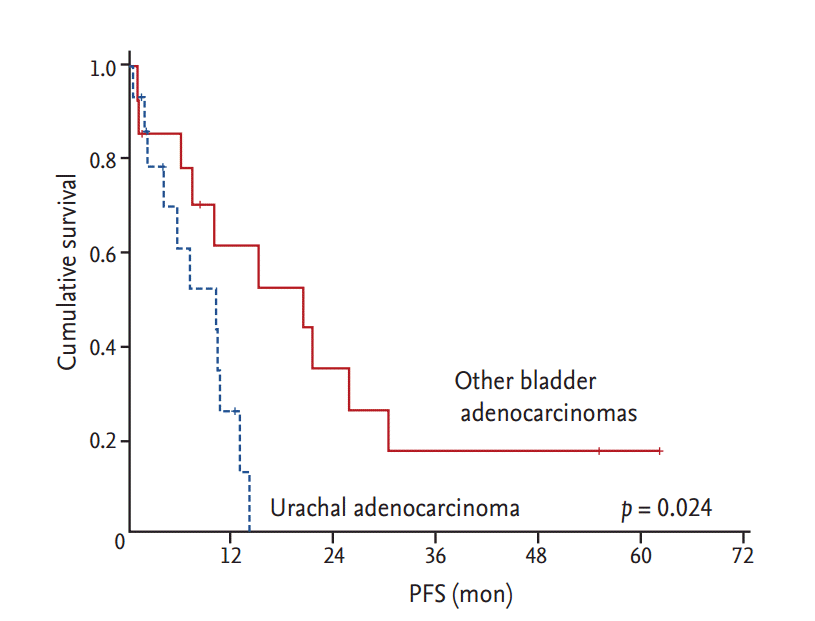
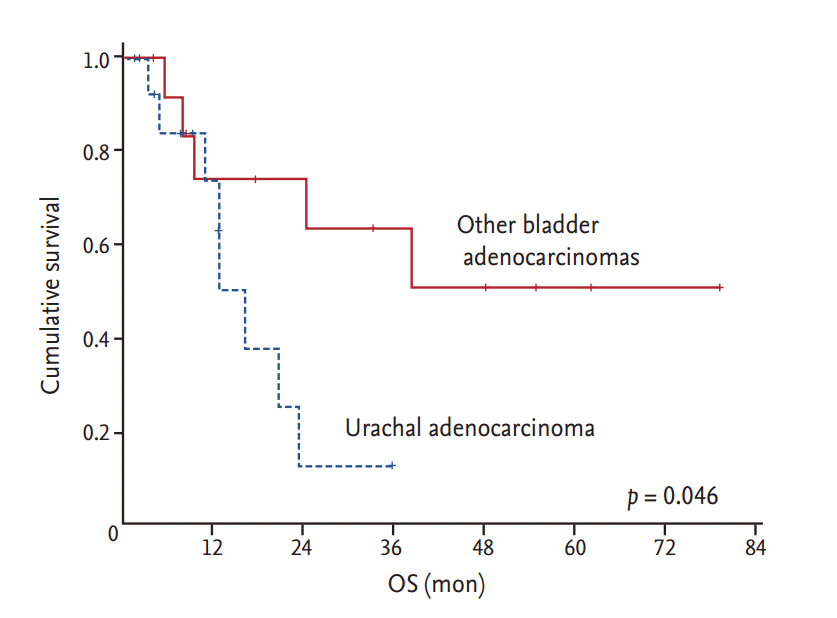
We observed that patients with nonurachal adenocarcinomas had a better median PFS of 20.6 months when compared with 10.4 months in patients with urachal adenocarcinomas (
p = 0.024) in the metastatic palliative chemotherapy setting. However, it remains unclear whether the survival of patients with urachal adenocarcinoma differs from that of patients with nonurachal adenocarcinomas itself. Wright et al. [
20] found that urachal cancer had a significantly better prognosis than nonurachal cancer. Compared to those with nonurachal tumors, patients with urachal adenocarcinoma were more likely to be younger (median age, 56 years vs. 69 years,
p < 0.001) and female (45% vs. 36%,
p = 0.02). Urachal lesions were less likely to be high grade (35% vs. 66%,
p < 0.001), but more likely to involve distant metastases (30% vs. 15%,
p < 0.001). While Mostofi et al. [
21] reported that urachal adenocarcinomas were associated with a significantly worse prognosis. The 17 tumors that were probably urachal in origin were characterized clinically by a more unfavorable course, all but three of the patients having died in less than 5 years. The poor prognosis of the urachal tumor is believed to be due to incomplete removal, for these tumors tend to spread along the remnants of the pre-existing urachal tract, not only in the bladder wall but in the anterior abdominal wall as well. Dandekar et al. [
22] and Zhang et al. [
9] stated the difference between urachal adenocarcinoma and nonurachal adenocarcinoma as not significant. These different results between studies about prognostic value of urachal and nonurachal adenocarcinoma might be depending on included different proportion of early stage, treatment option (operability), and comorbidity of patients.
This study has several limitations. This study was a retrospective analysis. Therefore it could have possibility of selection bias, insufficiency of medical records, and heterogeneity of treatment. The number of patients enrolled in the study was too small to evaluate efficacy of chemotherapy and analyze prognostic factors. Nevertheless, considering the relatively uncommon incidence of the disease, this is one of the largest data sets reporting clinical results of chemotherapy and could have an importance of histological types as a prognostic factor in advanced bladder adenocarcinoma.
Prospective trials are warranted to make a clinically meaningful improvement in palliative chemotherapy in the treatment of advanced bladder adenocarcinomas.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



