To the Editor,
The use of drug-eluting stents (DESs) that elute antiproliferative drugs locally during coronary artery interventions has increased in recent years because of their ability to inhibit neointimal proliferation. However, DESs may also affect the normal healing process of the vessel wall after vascular injury and may be associated with coronary artery aneurysm (CAA) formation in rare cases with various clinical presentations, from asymptomatic to sudden death and myocardial infarction resulting from stent thrombosis.
Despite its clinical significance, the incidence of CAA is very low. CAA is not detected by clinical evidence alone, and the natural course and mechanism of CAA are not yet proven. Furthermore, clinical reports regarding angiographic resolution of CAAs are very few and information is lacking.
Here, we present a case of CAA after sirolimus-eluting stent (SES) implantation and its angiographic spontaneous pseudo-resolution evaluated by serial intravascular ultrasound (IVUS).
A 42-year-old man was admitted to our hospital with resting chest pain. His medical history included hypertension on medication for 4 years, but no diabetes or smoking history. The clinical diagnosis was unstable angina, and elective coronary angiography was performed. Diagnostic coronary angiography revealed two-vessel disease, including a significant bifurcation lesion between the proximal-to-middle left anterior descending (LAD) artery and first diagonal branch, and a diffuse 87% diameter stenosis of proximal-to-distal left circumflex (LCX) artery.
The LAD bifurcation lesion was treated with the mini-crush technique using three SESs (Cypher Select+ , Cordis, Miami Lakes, FL, USA). Two SESs (3.5 ├Ś 33 mm and 3.0 ├Ś 33 mm) were implanted for the LAD lesion with an overlapping pattern, and a 2.5 ├Ś 23 mm SES was inserted for the diagonal lesion. Poststent adjuvant balloon dilation was performed at the LAD with a non-compliant balloon followed by kissing balloon inflation at the LAD and diagonal bifurcation. The proximal-to-distal LCX lesion was treated with 3.5 ├Ś 33 mm and 2.5 ├Ś 18 mm SESs.
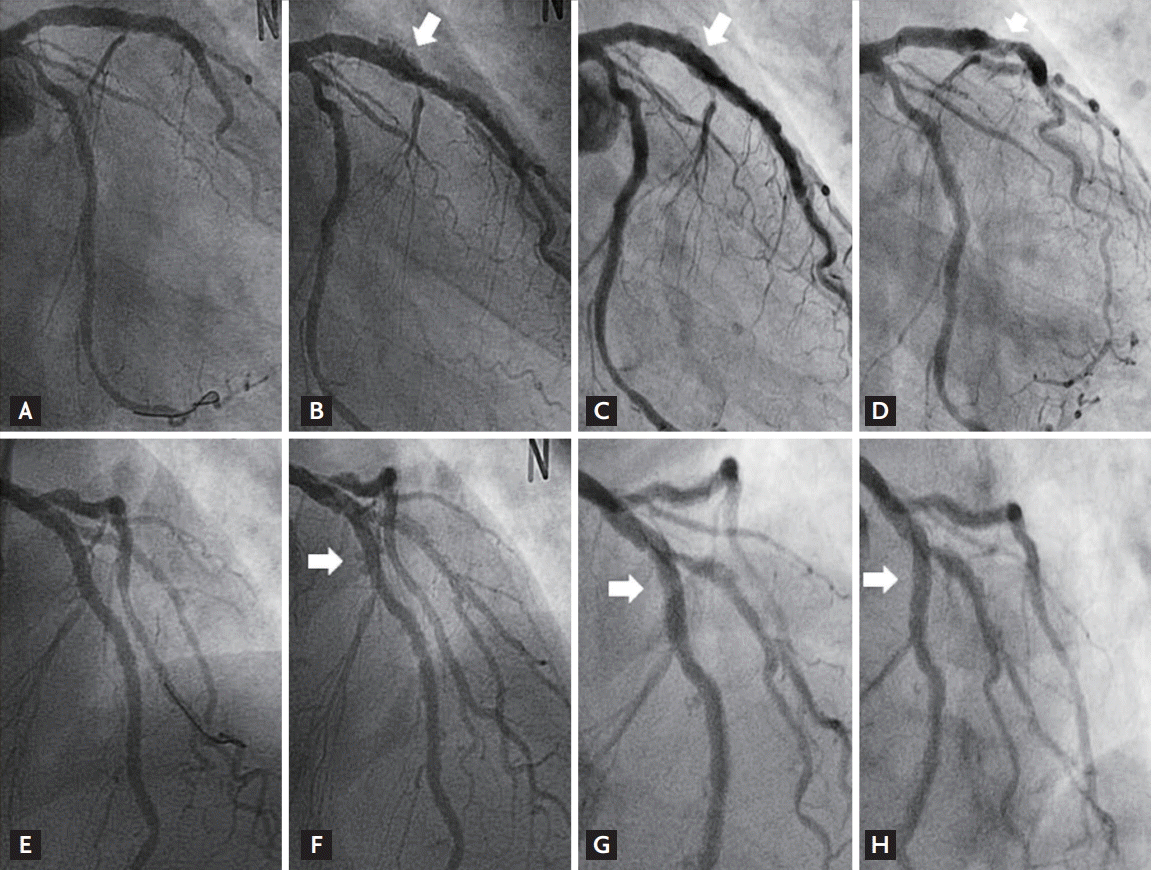
The procedural result was successful by angiogram (Fig. 1A and 1E), and IVUS (Galaxy 2, Boston Scientific Corp., Marlborough, MA, USA) revealed well-expanded stent struts without malapposition or dissection (Fig. 2A and 2E). The maximal external elastic membrane (EEM) and stent cross-sectional area (CSA) were 14.3 and 9.6 mm2 at the LAD, respectively. Aspirin, clopidogrel and cilostazol were maintained. One year later, follow-up coronary angiography showed multiple, diffuse, and saccular coronary aneurysms throughout the entire stented segments (Fig. 1B and 1F), accompanied with ectatic changes with malapposed stent struts noted by IVUS (Fig. 2B and 2F). The maximal EEM had increased to 45.3 mm2 without significant change of the stent CSA (9.5 mm2) compared with the post-stent IVUS CSA of the LAD at the index procedure.
Without additional intervention, the patient was followed carefully with continuation of dual anti-platelet treatments. For the next 2 years, the angiogram and IVUS were repeated to assess any changes in the CAA.
Angiography revealed partial, spontaneous resolution of the CAA compared with the previous examination (Fig. 1C, 1G and 1D, 1H). However, IVUS demonstrated remarkable findings, in which echogenic tissue had filled in the free space between the stent and EEM at the stented segments where the apparent angiographic improvement of the CAA occurred (Fig. 2C, 2G and 2D, 2H) without significant restoration of maximal EEM or change of stent CSA, with measured values of 45.2 mm2 and 9.5 mm2 at the LAD, respectively, compared with earlier examinations.
DESs have dramatically reduced the in-stent restenosis rates by suppressing neointimal hyperplasia. However, other complications of DESs and safety concerns, such as stent thrombosis, have been widely published.
Another less common complication of DES is CAA, defined as a dilatation that exceeds 1.5 times the diameter of the normal adjacent segment of the coronary artery. The incidence of CAA after DES implantation has been reported to range from 0.2% to 2.3% [1].
CAA after DES implantation is rare and may be detected mostly in asymptomatic patients, but is associated with restenosis, thrombosis and rupture in some cases [2]. Physical trauma to the stent, inflammatory reactions, such as local hypersensitivity (response to drug, polymer, or stent platform), delayed healing reactions (incomplete endothelialization over the DES), and incomplete stent apposition may contribute to CAA, but the exact mechanism and underlying pathophysiology remain unknown. Moreover, until recently, the natural history and clinical course of DES-related CAA had not been well-established [1].
There have been several case reports on spontaneous resolution of the CAA occurring after the insertion of paclitaxel-eluting stents [3]. To our knowledge, this is the first reported case of angiographic spontaneous pseudo-resolution of the CAA after SES, as well as an evaluation of the natural course by means of serial IVUS from the index procedure for 4 years.
The most important finding in the annual IVUS follow-up in our case was the discrepancy between coronary angiography and IVUS findings in terms of pseudo-resolution of the CAA. In this case, angiographic resolution did not result from restoration of the ectatic vessel, but from the filling up of the dead space between the stents and vessel wall with echogenic material. Thus, IVUS still revealed aneurysmal changes around the stented segments despite angiographic improvement of the CAA. The natural course of a CAA after DES implantation is unclear and can vary; however, stasis of blood and subsequent thrombus formation within an aneurysm might be a plausible mechanism of the spontaneous resolution of a CAA because a stable thrombus within a CAA could reduce dilating wall tension to the damaged vessel wall, which has reduced tolerance to intravascular pressure and prevents further dilatation of the CAA. Furthermore, these mechanisms may facilitate negative remodeling of the vessel wall and resolution of the CAA. Although we cannot clearly discuss the nature of the echogenic material that replaced the aneurysmal space in this case, we found that apparent angiographic resolution of a CAA does not always guarantee true restoration of the vessel, and filling up of the aneurysmal space with a thrombus or tissue could be an important mechanism of spontaneous pseudo-resolution of a DES-related CAA.
However, abluminal CAA thrombosis may constitute a double-edged sword, leading to complete CAA resolution in some patients, but increasing the risk of DES thrombosis in others. Continued clinical surveillance under prolonged dual antiplatelet therapy may be essential for CAA patients, even though follow-up angiography showed improvement of the CAA because the CAA was complicated by stent thrombosis, especially in patients in whom dual antiplatelet therapy is discontinued [4]. Moreover, serial studies are required to obtain mechanistic information of the natural course and related complications of CAAs.
High-resolution imaging modalities, such as optical coherence tomography (OCT), can provide more precise information on the surface findings and nature of the materials inside the CAA, as well as insights for determining further treatment plans, including the decision to discontinue dual antiplatelet therapy [5]. Unfortunately, OCT could not be performed in this case; however, considering its limited depth of penetration, both IVUS and OCT might be needed for a more complete evaluation of a CAA.
Eventually, the optimal treatment method can be determined based on long-term follow-up of CAA patients, which will provide a more accurate characterization of the consequences of DES-related CAAs. Until such data are available, a reasonable approach is to continue dual antiplatelet therapy and perform serial angiographic and intravascular imaging. An interventional sealing procedure or surgical treatment can be considered for patients with huge aneurysms or aneurysms that enlarge during follow-up.
In summary, we report the angiographic spontaneous pseudo-resolution of an SES-related CAA without true restoration of the vessel, as revealed by IVUS. Careful, long-term follow-up with high-resolution imaging modalities will be needed to establish the mechanism, course, and treatment of DES-related CAAs.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





