To the Editor,
Klinefelter syndrome (KS) is the most prevalent sex chromosomal anomaly in males [1]. To date, no significant association between KS and chronic myelogenous leukemia (CML) has been documented [2].
We present a case of CML in a patient with KS who demonstrated a persistent, suboptimal molecular response despite therapy with second-generation tyrosine kinase inhibitors (TKIs). Furthermore, he had a deletion of the argininosuccinate synthetase (ASS) gene at the 9q34 locus. He had no clinically significant comorbidity related to KS, which was diagnosed incidentally while performing bone marrow cytogenetics. It is unclear whether the presence of the derivative chromosome 9 deletion and sex chromosome anomaly affects the outcome in these patients with CML.
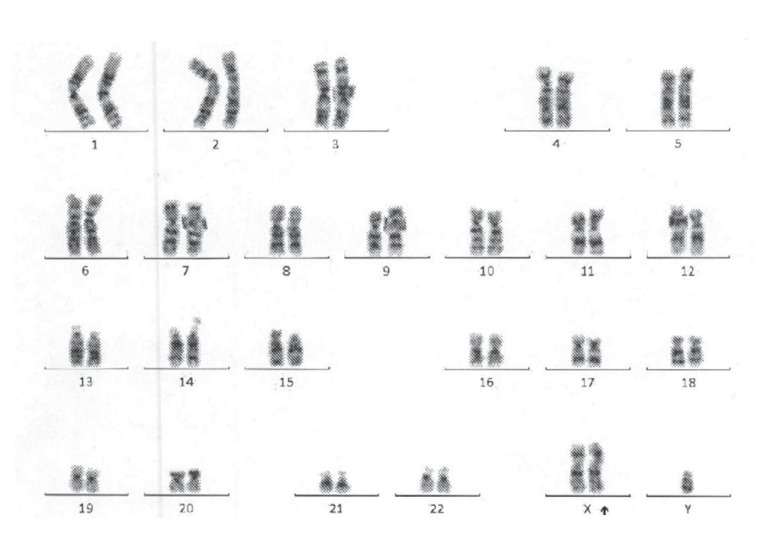
A 35-year-old Hispanic male was diagnosed with CML (in the chronic phase) in July 2009. He had a history of tuberculosis treated for 9 months in 1996 in Colombia. He initially presented with generalized fatigue, splenomegaly, and a high white blood cell count of 133,000/┬ĄL. Initial bone marrow biopsy revealed markedly hypercellular marrow with increased granulocytes at all stages of maturation and micromegakaryocytes. Few immature cells stained positive for CD34 and CD117. Initial cytogenetic studies showed 94% of cells with Breakpoint Cluster Region-Abelson (BCR-ABL) rearrangement on fluorescence in situ hybridization, 9q34.1 deletion and an extra X chromosome (Fig. 1). All of the 20 metaphase cells analyzed in cytogenetic studies showed an extracopy of an X chromosome and deletion of the ASS gene at 9q34 locus in bone marrow cells positive for BCR-ABL rearrangement.
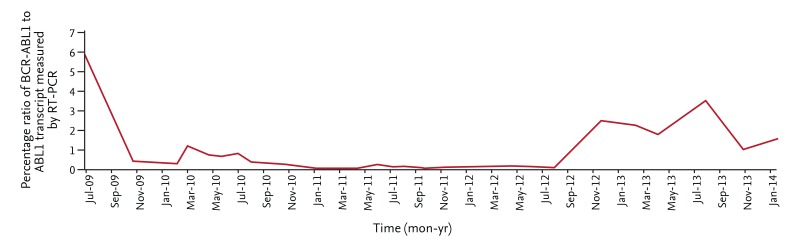
He was started on imatinib mesylate 400 mg daily. Subsequently, he achieved a complete hematologic response and a major molecular response in 3 months with 3-log reduction in BCR-ABL transcripts but only a minor cytogenetic response at 6 months. His medication history was further investigated, and it was found that he was taking rifabutin as prescribed by his primary care physician for a positive skin purified protein derivative test. Due to the drug interaction between imatinib mesylate and rifabutin, the dose of imatinib was increased to 800 mg daily in March 2010. Despite increasing the imatinib dose, reverse transcription followed by real-time polymerase chain reaction (PCR) of peripheral blood showed a persistent increase in BCR-ABL1 transcripts (Fig. 2). Dasatinib was subsequently started on June 2010 due to the persistent partial molecular response. In the interim, ABL kinase mutation analysis was performed and found to be negative. The patient was then evaluated for allotransplantation, but no related or unrelated matches were found. He was continued on dasatinib 100 mg daily. A complete cytogenetic response was finally achieved in April 2013. However, he continues to have a suboptimal molecular response with fluctuating levels of BCR-ABL1 transcripts in peripheral blood PCR studies.
Several case reports and cohort studies have suggested an increased risk for malignancies in males with KS. A recent British cohort study found a significantly increased risk and mortality from breast cancer, lung cancer, and non-Hodgkin lymphoma in males cytogenetically diagnosed with KS [2]. In the same study, an increased incidence of leukemia was also noted, but the difference was not statistically significant.
In the past, few cases of Philadelphia-chromosome-positive CML have been reported in males with KS [1,3]. In a study by Alimena et al. [3] on the cytogenetics of leukemic cells in patients with constitutional chromosomal anomalies, it was observed that acute leukemia occurred predominantly in patients with trisomy 21, whereas chronic myeloproliferative disorders were dominant in those with balanced translocations and sex chromosome anomalies, including KS. All of the patients presented with CML in the chronic phase, with the exception of one case reported by Toubai et al. [1] in which the patient presented in blast crisis and subsequently underwent allogeneic bone marrow transplantation from an unrelated donor. In our case, the patient presented in the chronic phase with bone marrow cytogenetics showing 47, XXY, t(9;22)(q34;q11) with deletion of ASS at 9q34.1.
It is unclear whether the presence of KS has any prognostic significance in patients with CML. The patient presenting with blast crisis reported by Toubai et al. [1] relapsed after bone marrow transplantation and subsequently died due to disease progression. Our patient has a persistent suboptimal molecular response despite therapy with second-generation TKIs.
Some controversy exists regarding the prognostic significance of large deletions at the t(9; 22) breakpoint in patients with CML. Quintas-Cardama et al. [4] reported a study of 352 patients with CML and found similar rates of cytogenetic response, overall survival and response duration with imatinib mesylate in those with or without derivative chromosome 9 deletion. Huntly et al. [5] reported lower rates of hematologic and cytogenetic responses in patients with deletions when treated with imatinib mesylate. To date, no study of the rates of molecular remission in patients with derivative chromosome 9 deletions and the effect of second-generation TKIs, if any, on survival and rates of remission (hematologic, cytogenetic, and molecular) in this subgroup of patients has been conducted.
To our knowledge, this is the first case report of CML in a patient with KS to address the outcome and response to TKIs. Our patient had a persistent suboptimal molecular response with fluctuating BCR-ABL activity in peripheral blood detected by PCR, despite therapy with second-generation TKIs. Further studies are required to identify the prognostic significance of KS and derivative chromosome 9 deletions in patients with CML.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





