See Article on Page 403-409
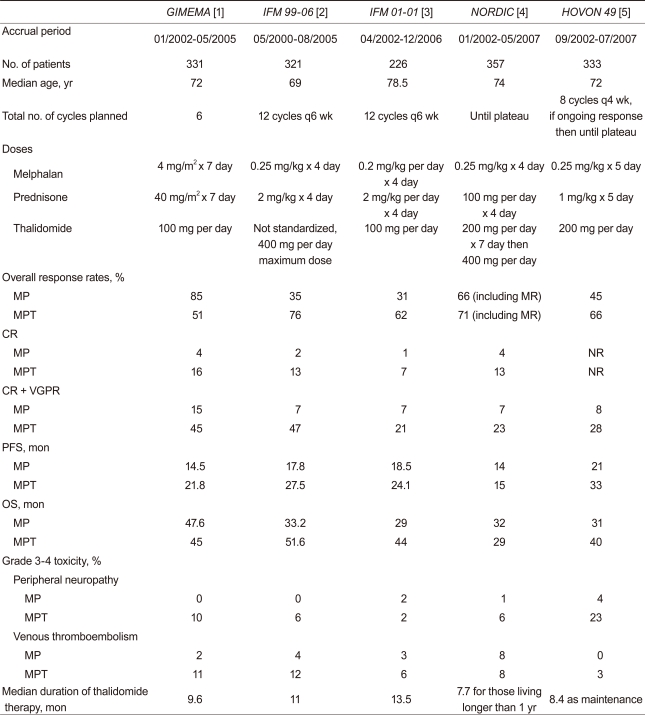
The standard of care for patients with multiple myeloma (MM) who are not eligible for high dose therapy + autologous stem cell transplantation (aged > 65 years or < 65 with severe comorbidities) has been for many years the combination of melphalan + prednisone (MP). In recent years, the novel drugs thalidomide, lenalidomide and bortezomib have been incorporated in the abovementioned 'old' regimen. It is becoming increasingly clear that addition of an individual novel agent to the traditional MP regimen could be synergistic and influence the disease outcome. Consequently, the appropriateness of the use and tolerability of such regimens in different patient populations is another area of active research interest. Five randomized studies comparing the MP plus thalidomide (MPT) regimen with MP alone have provided conflicting evidence [1-5]. Although there is greater agreement with regard to superior response rates (RRs) with MPT to MP, the impact on progression free survival (PFS) and overall survival (OS) is less clear, with some trials demonstrating an improvement in PFS and/or OS with MPT and others showing no difference in outcomes.
Importantly, the dose intensities of all three drugs (M, P, and T) differed among the five trials (Table 1). The optimal dose of thalidomide as an anti-myeloma therapy is not yet known, mainly because its exact mode of action is unclear. While traditional cytotoxic drugs show a dose-response relationship within the therapeutic range, a similar correlation cannot necessarily be presumed to occur with immunomodulatory drugs. Thalidomide appears to be immunomodulatory as well as anti-angiogenic. Both these activities may contribute to the anti-myeloma effect of the drug, but the immunomodulatory action is thought probably to be the major contributor [6]. It has been suggested that a threshold level of the active drug may need to be achieved to 'switch on' the immunomodulatory and, hence, the major anti-neoplastic mechanism, with further dose increases failing to enhance anti-neoplastic activity [7]. Moreover, pharmacokinetic data on thalidomide use in humans are not well-characterized and show considerable individual variation. The optimal dose probably needs to be individualized.
Among the above five studies, melphalan and thalidomide doses were lowest in the IFM 01-01 study (involving patients above 75 years of age), yet it demonstrated a survival benefit [3]. The Nordic group demonstrated that better tolerated, lower thalidomide doses (100-200 mg) were sufficient and comparable to higher doses (400 mg) used in the induction phase, and 200 mg in the maintenance phase [4]. Unfortunately, thalidomide has many side-effects, some of which are dose-related. Better RRs with such high doses did not translate into survival benefit in the MPT arm. Nearly a third of patients had discontinued treatment by three months, and stopping of therapy increased to 56% by the end of the first year. Furthermore, during the first six months of treatment, the Nordic study reported 35 deaths in the MPT arm, 23 in patients older than 75 years [4].
In this issue of the Korean Journal of Internal Medicine, Dr. Chang and colleagues [8] report that lower dose thalidomide (50 mg per day) plus MP is effective in my MM, with 57.1% overall RRs and 23.8% complete RRs. Although MP doses with four-week cycles of melphalan (4 mg/m2) and prednisone (40 mg/m2) on days 1-7 are active in untreated MM, the daily doses used were low, and unlikely to produce such high response rates. This is supported by others who reported ultra low doses of thalidomide 25 mg or 50 mg on alternate days, respectively [7,9]. One of the major implications of this study lies in the higher than expected RRs, despite the lower dose of thalidomide. There is not a clear dose-response relationship between thalidomide and myeloma response using dose ranges of 50-400 mg/day [10]. However, interpretation of these results should be approached with caution. An explanation for the high observed RRs with MP plus thalidomide 50 mg may be that the cohort represented a selected group who metabolized thalidomide slower than the average. The small number of patients enrolled in this study may have achieved adequate plasma levels of the active thalidomide metabolites, despite taking low doses. This study showed substantially similar RRs; however, it also showed a shorter response duration than that seen in the IFM 01-01 trial [3]. The reason for the shorter PFS in this study may be that the patients were treated for a median four cycles of MPT (range, 1 to 12).
A reduced dose of thalidomide with MP has the potential to decrease adverse effects and does not have to require dose reduction, as compared to conventional MPT. With respect to the withdrawal rate from the IFM 01-01 trial, 48 patients (42.4%) discontinued treatment due to toxicities, while two (10%) discontinued treatment in this study. However, grade 3-4 peripheral neuropathy was reported in one (5%) patient, which is comparable to other studies (Table 1). Moreover, the occurrence of venous thromboembolism (VTE) was reported in 10% of patients in this study, but one unexplained death might be considered a composite adverse effect, suggesting that the percentage of VTE (approximately 15%) was substantially higher than in other studies (Table 1). The fact that the incidence of grade 3-4 adverse events was not significantly reduced in patients who received low-thalidomide dose MPT is inconsistent with the lower discontinuation rate in this study.
During the past 10 years, dramatic changes have occurred in the treatment options for MM. The challenge now is to build on this progress and find new, more active, and less toxic agents and combinations. MP plus thalidomide 50 mg may be considered one of the new combination regimens for elderly and/or transplant ineligible MM patients. Randomized trials of varying thalidomide doses in patients receiving MPT would further advance the field and facilitate treatment tailored to the individual patient.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





